Against the backdrop of the global energy transition, lithium-ion batteries, as the core components of electric vehicles and energy storage systems, have made performance evaluation critically important. Battery testing encompasses multiple items such as capacity, internal resistance, and cycle life. Cyclic voltammetry (CV), as an important electrochemical analysis technique, can deeply reveal the intrinsic characteristics of battery materials.
By analyzing the current-voltage curve, CV testing provides researchers with key information to evaluate the reaction mechanism, reversibility, and stability of electrode materials. It is hailed as the "performance decoder" and "electrochemical electrocardiogram" of electrode materials.
Principle and significance of CV testing: The "performance decoder" of battery materials
Cyclic voltammetry is an analytical method that studies electrode processes by applying a triangular waveform potential and measuring the response current. Its basic principle involves applying a linearly time-varying triangular wave potential between the working electrode and the reference electrode, and recording the relationship curve between the current obtained at the working electrode and the applied voltage.
During the CV test, when the electrode potential gradually shifts negatively near the equilibrium potential, the reactant begins to reduce on the electrode, generating a Faradaic current. As the potential becomes more negative, the concentration of the reactant on the electrode surface gradually decreases, and the current increases to a maximum before gradually declining. During the reverse scan, the electrode potential gradually shifts positively, the product particles begin to oxidize, and the current increases to a peak oxidation current before decaying again.
Several important parameters can be obtained from a cyclic voltammogram: the anodic peak current (Ipa), the cathodic peak current (Ipc), the anodic peak potential (Epa), and the cathodic peak potential (Epc). The method for determining Ip is as follows: extrapolate a tangent line along the baseline to a point under the peak, then drop a perpendicular line from the peak top to this tangent; the height between these points is Ip (Figure 1). Ep can be read directly from the horizontal axis at the point corresponding to the peak top.
Generally, for a reduction peak (the upward peak), a more positive peak potential and a larger peak current indicate that the species is more easily reduced. For an oxidation peak (the downward peak), a more negative peak potential and a larger peak current indicate that the species is more easily oxidized. For a simple electrode reaction process, the criteria for judging the degree of reversibility of the electrode reaction are:
Ipa = Ipc (Ipa, Ipc are proportional to V1/2)
Epa - Epc ≈ 60 mV (under 25°C conditions)
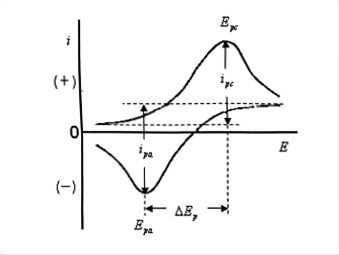
Figure 1 typical cyclic voltammetry curve
The data provided by the CV curve enable researchers to analyze the reversibility of the electrode reaction, study the mass transfer mechanism to understand reaction kinetics, and assess the stability of the material. These parameters provide an important basis for evaluating the performance of electrode materials.
The significance of CV testing lies in its ability to function like a "comprehensive health monitor," providing a full assessment of various performance indicators of electrode materials. From redox characteristics to reaction reversibility, and from mass transfer mechanisms to material stability, CV curves offer rich information that guides researchers in optimizing battery material systems.
CV Testing equipment requirements: The hardware support behind accurate data
Obtaining high-quality CV test data relies on high-performance equipment support. The CV testing system must possess multiple precision characteristics to ensure the accuracy and reliability of the measurements.
High-precision voltage and control capability are fundamental requirements for CV testing equipment. The device must be able to accurately output a triangular wave potential and maintain a linear change in potential during the scanning process. Voltage control accuracy directly affects the shape of the CV curve and the accuracy of the characteristic parameters.
Simultaneously, current measurement sensitivity is also crucial. Since the Faradaic current generated by the electrode reaction is usually small, the equipment needs the capability to detect weak currents. Especially when studying low-concentration systems or trace active substances, a high-sensitivity current detection system ensures that even tiny redox peaks can be accurately captured.
A wide adaptability of the scan rate range is also an important feature of high-performance CV testing equipment. CV tests at different scan rates can provide different information—low scan rates (e.g., 0.1 mV/s) are suitable for studying diffusion-controlled reactions, while high scan rates help analyze reaction kinetics. The equipment should provide precise scan rate control over a wide range.
Low noise and high stability are guarantees for obtaining reliable data. Electrochemical tests are sensitive to environmental interference; excellent equipment design needs to minimize external disturbances to ensure test result reproducibility. The NEWARE CT-8002S possesses excellent performance, fully meeting research-grade demands.

Figure 2 NEWARE CT-8002S-5V100mA-CV
Data processing and analytical capabilities are also important considerations for modern CV testing equipment. Advanced equipment can not only collect data but also assist in preliminary analysis, such as automatically identifying peak potentials and peak currents, and even calculating diffusion coefficients using the Randles-Sevcik equation. These functions greatly improve research efficiency.
Conclusion
CV testing is a key tool for analyzing battery reaction mechanisms. Its value depends on high-precision equipment—including voltage/current control accuracy, a wide range of scan rates, system stability, and intelligent data analysis capabilities—which collectively ensure data accuracy and scientific research value.
Supplement: Some of the information presented above was obtained from the Internet. We are very sorry if there is any infringement! You can contact us for deletion!