Introduction
With the continuous development of electrochemical research techniques, the combination of in situ testing methods and CV measurements has opened up new pathways for battery research. This coupled technology allows researchers to monitor other physical and chemical changes inside the battery in real-time during CV measurements, obtaining more comprehensive dynamic information about the battery.
Various in situ coupling techniques have been developed to enhance the analytical capabilities of CV testing. In recent years, operando methods have integrated CV measurements with other experimental techniques to better characterize battery systems.
"OST-SRTM"
Fiber optic devices have been integrated into commercial battery cells to monitor volume, temperature, and chemical changes during CV measurements. A research team from Tsinghua University developed an in situ spatiotemporal super-resolution thermal monitoring system named "OST-SRTM" [1]. This technology uses an Archimedean spiral optical fiber implanted inside the battery to draw a two-dimensional temperature field of the battery interior in real-time, achieving an ultra-high resolution of 1820 points per cm² at a rate of one frame every 3 seconds. Research revealed that unprotected lithium metal batteries can develop local high-temperature zones ("hot spots") exceeding 15°C during cycling, which is a precursor to thermal runaway caused by dendrite growth. Furthermore, fiber optic Fabry-Perot cavity sensors can monitor the internal gas pressure of batteries. Studies using this technique measured that the internal pressure during thermal runaway can reach up to 2 MPa, and the pressure relief valve only opens when the internal temperature surges to nearly 510°C.
In the figure below, Figure 1d shows the cyclic voltammetry curve of the battery during the 48th cycle. By coupling CV testing with other tests, the changes in battery voltage, capacity, and temperature are analyzed to establish correlations between these varying parameters.
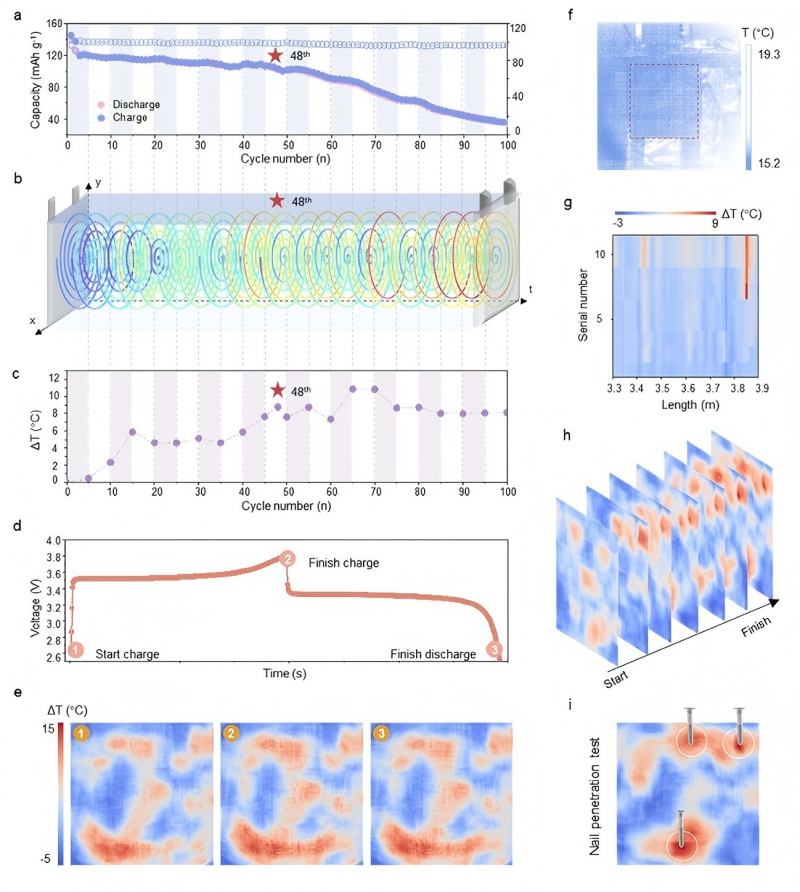
Figure 1 electrochemical properties and spatiotemporal temperature distribution throughout the lifecycle of lithium metal batteries, along with the pinning test [1]
Other methods
Other methods, such as Nuclear Magnetic Resonance Microscopy (NMR) [2] and Transmission Electron Microscopy (TEM) [3], have also been used to probe local structure and chemical kinetics during cycling.
CV-PWP
The CV-PWP coupling technique is an operando characterization method designed to synchronously capture both the electrochemical signals and internal mechanical stress changes in a battery during operation.
CV Component: By applying a cyclic voltage scan, it monitors the redox reactions, phase transition processes, and side reactions occurring inside the battery. This helps us understand the battery's "chemical behavior."
PWP Component: Its core lies in probing the electrostatic charge distribution inside the battery. During battery cycling, the intercalation and deintercalation of lithium ions cause volume changes in the electrode materials, subsequently generating internal stress. By monitoring the resulting pressure wave propagation, PWP provides information on the microstructural changes and mechanical stability of the electrode materials, and may even allow inference of ion migration. This reveals the battery's "physical behavior."
The advantage of this technique is its ability to directly correlate specific electrochemical events (e.g., a peak in the CV curve) with simultaneous physical changes (e.g., a sudden stress change), providing a more comprehensive perspective for understanding battery operating mechanisms.
It can be applied in the following scenarios:
Monitoring Stress Evolution in Silicon Anodes during Cycling: During lithium ion intercalation (near the reduction peak) and deintercalation (near the oxidation peak), intense pressure wave signals should be observable. Analyzing the amplitude attenuation or waveform changes of the pressure wave signals can indicate mechanical failures like electrode material cracking or SEI film fracture.
Evaluating Wetting and Ion Transport in High-Loading Electrodes: Uneven electrolyte distribution or hindered ion transport may manifest as significant polarization in the CV curve, while specific patterns of noise or signal delay might appear in the PWP signal.
Probing Solid-State Battery Interface Stability and Dendrite Growth: The nucleation and growth of lithium dendrites can cause local stress changes. Abnormal precursors might appear in the PWP signal before macroscopic short circuits occur.
EIS-CV
The EIS-CV coupling framework is another promising in-situ analysis technique. Its power lies in providing complementary and mutually verifying information: EIS is more adept at quantitatively analyzing the individual components of interfacial processes (e.g., charge transfer resistance, double-layer capacitance), revealing the "state" of the interface. CV more intuitively reflects the overall electrochemical activity and reaction reversibility, showing the "behavior" of the interface.
EIS-CV can be applied to diagnose performance drift in electrochemical sensors. In a study targeting the performance degradation of electrochemical sensors (Figure 2), the combination of EIS and CV revealed distinct "aging" processes in different electrode materials [4].
For unmodified carbon electrodes subjected to continuous CV cycling, EIS detected a significant decrease in charge transfer resistance, while the separation between the redox peaks in the CV curve decreased. Together, this indicates the electrode surface undergoes an "electrochemical polishing" effect, removing the original passivation layer and exposing more active sites, thereby improving performance. Pt/C modified electrodes exhibited a more complex trend. EIS parameters showed their charge transfer resistance first decreased and then increased, and the effective capacitance first increased and then decreased. This indicates their performance experienced a brief improvement followed by degradation due to Pt nanoparticle dissolution and carbon support oxidation. A subsequent decrease in current response was also observed in the CV curves, corroborating the EIS findings.
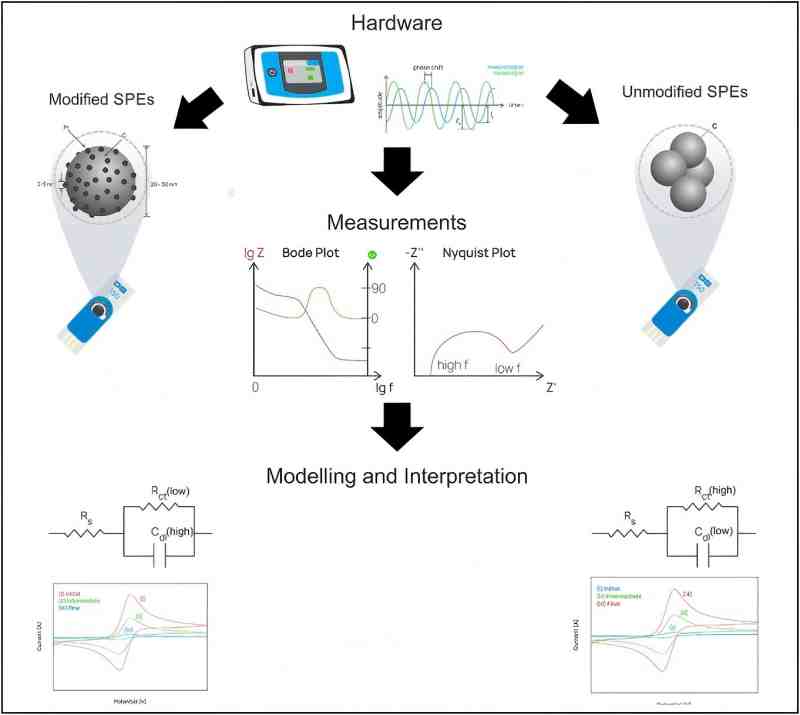
Figure 2 schematic diagram of the diagnostic workflow for in situ EIS-based sensor performance evaluation (SPEs) [4]
EIS-CV can also be used in research related to optimizing electrodes via plasma treatment. One study demonstrated how oxygen plasma treatment can efficiently optimize screen-printed carbon electrodes [5]. EIS data showed that the charge transfer resistance of the treated electrode decreased sharply, indicating a significant reduction in the barriers to ion and electron transport at the electrode interface. Meanwhile, the CV curve visually demonstrated the enhancement in electron transfer kinetics, manifested as a narrowing of the separation between the redox peaks. Through EIS and CV, this study clearly revealed how surface modification techniques can fundamentally enhance electrode performance.
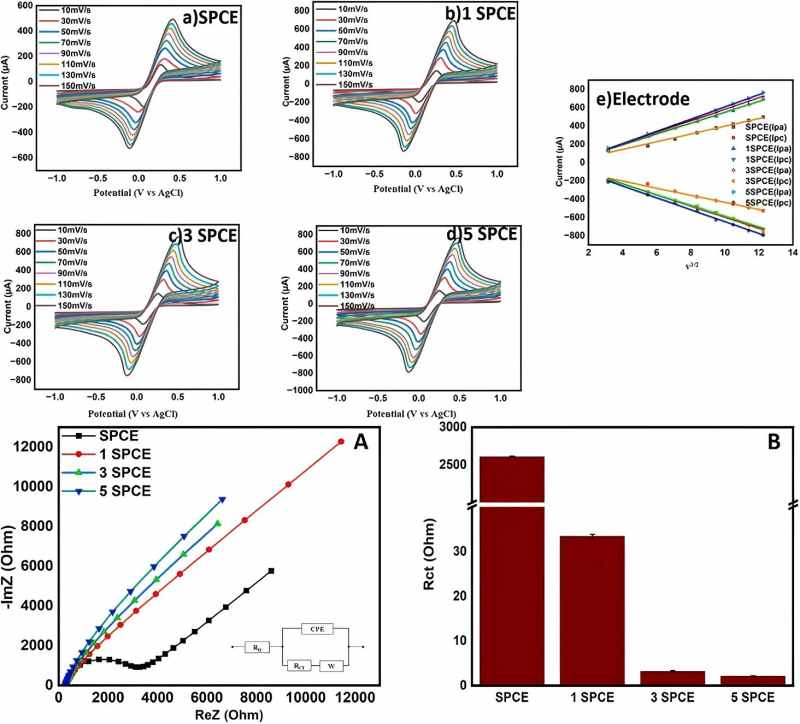
Figure 3 CV tests and EIS tests of SPCE, 1SPCE, 3SPCE, and 5SPCE [5]
Conclusion
The advantages of coupling in situ testing with CV are evident: it provides real-time, dynamic internal battery information, allowing researchers to directly observe material evolution processes during battery operation; it achieves synchronous multi-parameter analysis, enabling the simultaneous acquisition of chemical, physical, and electrochemical information; and it possesses in-depth mechanistic analysis capabilities, offering a new perspective for understanding complex electrochemical processes. This multi-technique fusion approach represents the forefront of electrochemical research, transforming traditional "black box" battery testing to a new level of real-time visualizable analysis.
References
[1] Zhang C, Liu Z, Lao Z, et al. Operando spatiotemporal super-resolution of thermal events monitoring in lithium metal batteries[J]. National Science Review, 2025, 12(5): nwaf088.
[2] Singer R, Hu W, Liu L, et al. Non-Invasive Regional Neurochemical Profiling of Zebrafish Brain Using Localized Magnetic Resonance Spectroscopy at 28.2 T[J]. Molecules, 2025, 30(21): 4320.
[3] Hu H, Yang R, Zeng Z. Liquid-phase TEM study of electrochemical reactions at multiple interfaces[J]. Matter, 2025, 8(3).
[4] Krishnamurthy A, Soderžnik K Ž. Multivariate diagnostics of electrochemical sensor drift by in situ impedance spectroscopy and voltammetry: A benzenediol-based framework[J]. Sensing and Bio-Sensing Research, 2025, 50: 100871.
[5] Luhar S, Sadowska K. Plasma-Tailored SPCEs for Enhanced Surface Reactivity and Electron Transfer: Toward Improved Electrodes[J]. Surfaces and Interfaces, 2025: 107943.