Lithium-ion batteries in Evs: advantages, market dominance and thermal runaway safety risks
Global energy policies are progressively shifting from fossil fuels to renewable sources. As crucial energy storage devices, lithium-ion batteries hold significant advantages over other battery technologies in the field of electric vehicles (EVs). In recent years, continuous improvements in the energy density of lithium batteries, coupled with advancements in fast-charging technology, have enabled EVs to be recharged in very short times. Hydrogen fuel, once promoted for its rapid refueling capability, can no longer compete with lithium batteries in the EV market [1]. Consequently, a growing number of consumers are choosing to purchase new energy electric vehicles.
However, higher energy density often comes at the cost of reduced battery stability [2]. Compounded by shrinking profit margins leading some manufacturers to cut corners on safety standards in EVs [3], the number of reported EV fire incidents has increased in recent years. As thermal runaway is a critical safety concern for lithium batteries that cannot be overlooked, it has attracted significant attention from researchers worldwide.
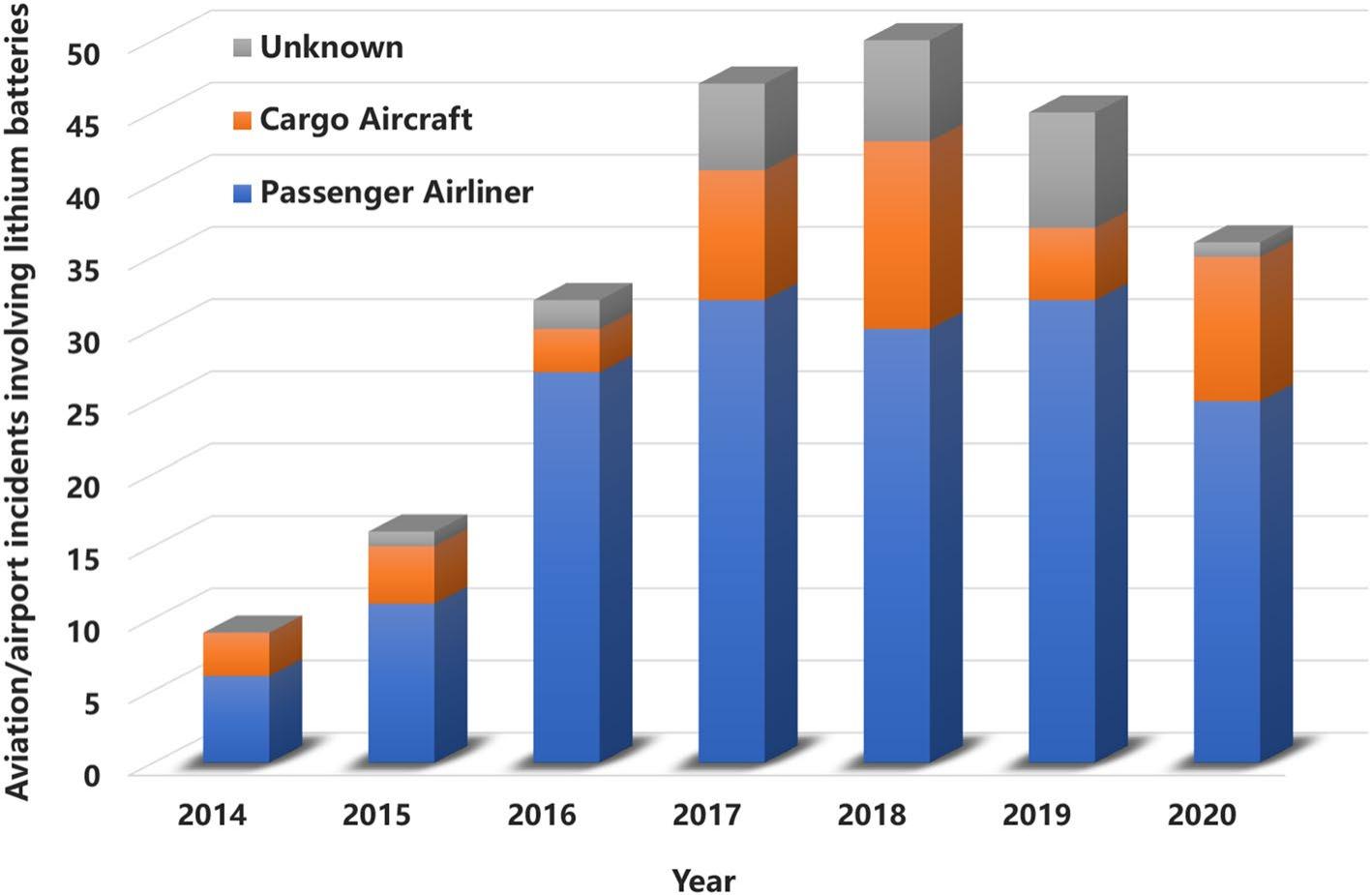
Figure 1 aviation/airport incident data involving lithium batteries recorded from 2014 to november 4, 2020 [4]
Challenges of lithium batteries
Compared to lithium-metal batteries, lithium-ion batteries exhibit less lithium dendrite formation and demonstrate superior cycling stability. However, in practical applications, when lithium-ion batteries encounter abnormal conditions such as extreme temperatures, overcharging/over-discharging, or material compression, they may lose the ability to function independently. This can potentially lead to short circuits and ultimately trigger thermal runaway [5].
Heat sources in lithium batteries
The working principle of batteries is based on chemical reactions, and the heat generated is related to the electrode materials and the reaction equations. Researchers [6] have proposed that battery management systems must account for the heat generation processes involving both reversible and irreversible heat. The irreversible heat originates from the Joule effect, generated by charge transfer within the current collectors, while the reversible heat stems from chemical reactions. The reversible heat generated varies with different electrode materials; for instance, graphite/LFP batteries produce significantly less reversible heat than graphite/LiCoO2 batteries [6]. Consequently, data indicate that graphite/LFP batteries possess better thermal stability.
Factors determining reversible heat sources
Positive and Negative Electrode Materials: Different electrode materials determine the type of chemical reaction, thus the choice of electrode material significantly impacts the heat generated.
Battery Residual Energy, State of Charge (SOC), Energy Released by the Battery, and Depth of Discharge (DOD) Cycles: These parameters are related to the lithium-ion diffusion rate. Therefore, preventing overcharging and over-discharging can effectively avoid thermal runaway.
Internal Battery Temperature: As the internal temperature of the battery increases, irreversible side reactions can be triggered within the battery, generating additional heat.
Factors determining irreversible heat sources
Charge/Discharge Current: According to Q = I2Rt, a larger current leads to more heat generated by the Joule effect. The resistance includes ohmic resistance, which is the intrinsic resistance of the battery components (including the anode, cathode, and electrolyte materials), and electrochemical resistances, such as the resistance encountered during lithium-ion diffusion through various battery components, and the charge transfer interfacial resistance at the electrolyte/electrode material interface.
Thermal runaway process
The thermal runaway of lithium-ion batteries is generally divided into four stages:
Stage 1: Trigger and Heat Accumulation (80-90℃), - Initial Heat Generation: Heat begins to generate inside the battery. If the rate of heat generation exceeds the battery's heat dissipation capacity, the temperature starts to rise.
Stage 2: Chain Reaction Begins (90-150℃), SEI Layer Decomposition: When the temperature rises to about 90-120℃, the protective film (SEI layer) on the anode surface decomposes exothermically. The exposed fresh anode reacts more violently with the electrolyte, continuing to generate heat. Separator Melting (130-150℃): The separator begins to melt and shrink, causing large-scale contact between the positive and negative electrodes, leading to severe internal short circuits and instantly generating massive heat. This is a critical step pushing towards runaway.
Stage 3: Reaction Intensifies (150-250℃), Cathode Material Decomposition: When the temperature reaches approximately 150-250℃, cathode materials, especially high-energy-density ternary materials (NCM/NCA), begin to decompose, releasing oxygen. Electrolyte Decomposition and Reaction: The electrolyte itself decomposes at high temperatures and reacts violently with the anode and cathode, producing large amounts of flammable gases (such as hydrogen, carbon monoxide, alkanes).
Stage 4: Complete Runaway (>250℃), Combustion and Explosion: The accumulated flammable gases mix with the oxygen released from the cathode and ignite at high temperatures, causing the battery to eject flames. If the battery casing ruptures due to excessive internal pressure, a physical explosion can occur. Thermal Propagation: The thermal runaway of a single cell can release extremely high temperatures (exceeding 800℃), sufficient to ignite adjacent cells, causing thermal runaway throughout the entire battery pack or system.
Causes of thermal runaway
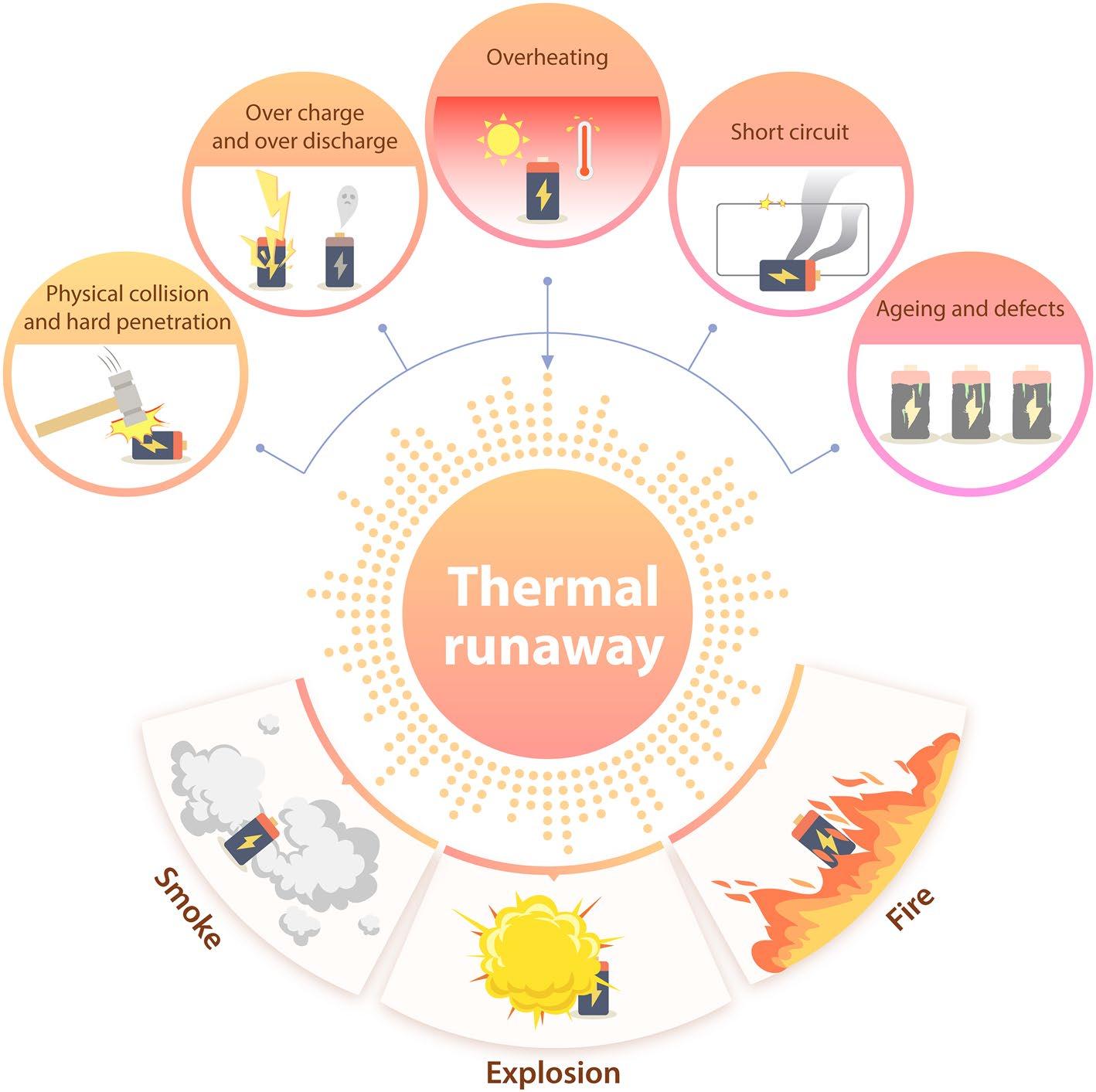
Figure 2 Causes and Hazards of Lithium Battery Thermal Runaway[7]
Physical Impact and Hard Penetration: When lithium-ion batteries undergo severe physical impact or hard penetration, the battery system becomes susceptible to deformation, potentially leading to separator tearing, internal short circuits, and even electrolyte leakage. The substantial heat generated by persistent short circuits can potentially cause fires.
Overcharging and Over-discharging: When a battery remains connected to a power source for an extended period, excessive energy continuously flows into the battery. This sustained charging can cause the battery to generate excessive heat, subsequently triggering electrolyte decomposition and gas production. Concurrently, internal battery pressure increases, structural deformation occurs, and performance deteriorates sharply. Compared to normal charging, the heat generated during overcharging is more destructive than during over-discharging, due to additional side reactions and increased internal resistance.
Overheating Phenomenon: Thermal runaway can occur when a battery overheats due to usage beyond its normal operating limits. The thermal effects can lead to the melting of the separator, decomposition of electrodes and electrolyte, among other side effects. These multiple side effects, in turn, intensify the exothermic reactions within the battery, ultimately resulting in thermal runaway.
Short Circuits: Short circuits can be categorized into external and internal short circuits. An external short circuit occurs when the different electrodes are connected by a conductor. External short circuits are primarily triggered by factors such as battery impact or water immersion. To mitigate the risk of external short circuits, protective electronic devices can be employed to cut off the high-current power source. The most commonly used protective components include fuses, Positive Temperature Coefficient (PTC) components, magnetic switches, and bimetallic thermistors. An internal short circuit occurs when separator failure leads to direct contact between the positive and negative electrodes. Once triggered, intense chemical reactions spontaneously occur inside the battery, not only causing battery failure but also releasing a significant amount of heat, which can easily lead to fire or explosion. Internal short circuits are the most probable trigger for thermal runaway; nearly all thermal runaway cases ultimately involve an internal short circuit phenomenon. Their causes are complex and varied, including physical impact causing separator tearing, sustained internal temperature rise leading to separator melting, and lithium dendrite penetration of the separator at the anode deposition sites. The most direct and effective method to prevent thermal runaway is to eliminate internal short circuits.
Battery Aging and Defects: Battery aging and internal defects not only affect its thermal performance but also lead to capacity fade and power degradation. Battery aging can be divided into calendar aging and active aging. The former stems from long-term high-temperature storage, while the latter is related to the long-term usage of the battery. Both types of aging alter the performance characteristics of the battery. If the battery has internal defects (such as poor manufacturing quality, inferior separator quality, or material contamination), it may lead to battery failure or even thermal runaway.
Improvement methods
In response to the various causes of lithium battery thermal runaway, several improvement methods have been developed (Figure3).
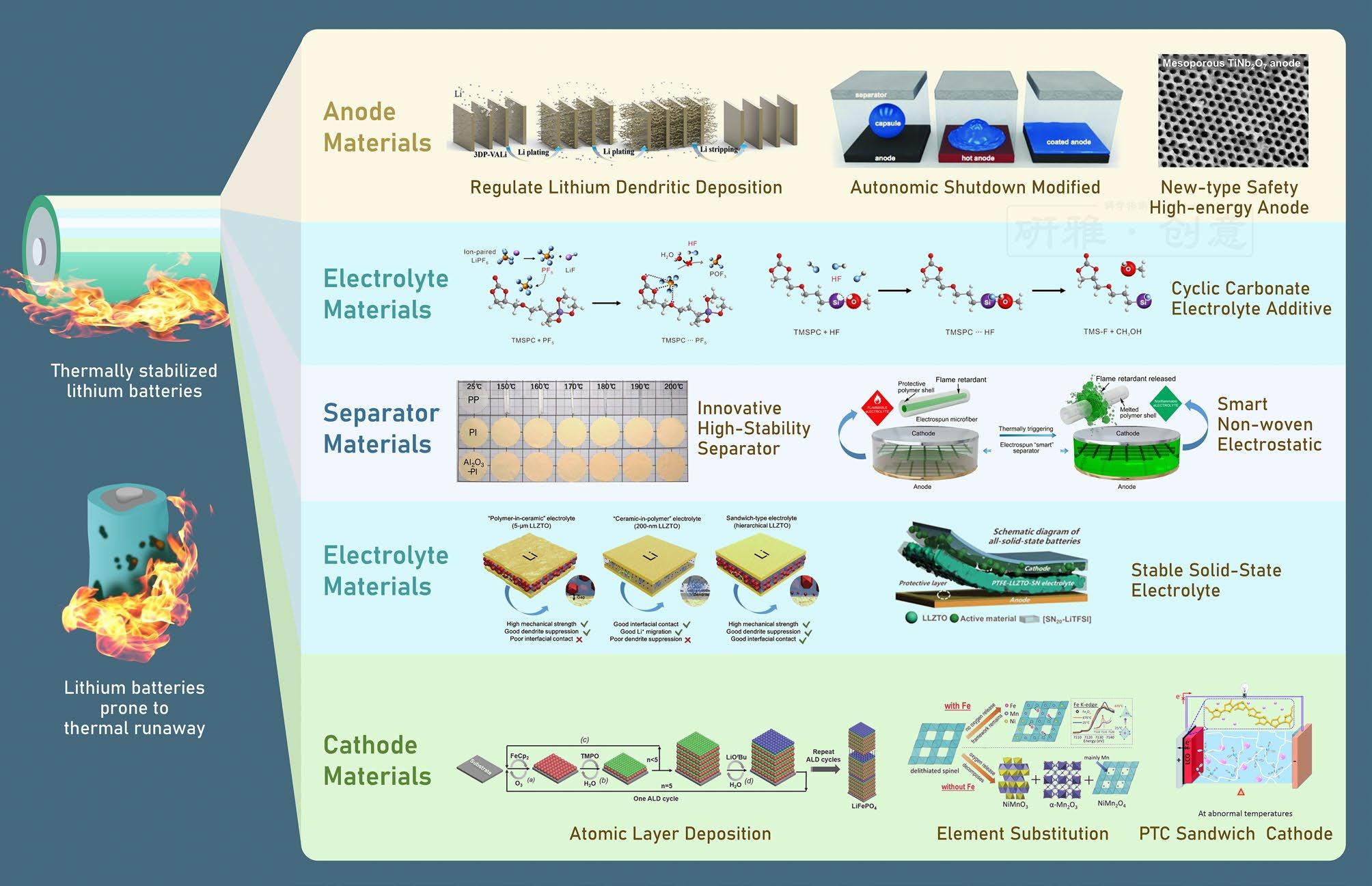
Figure 3 Improvement Methods for Addressing Lithium Battery Thermal Runaway[7]
Anode materials:
The core strategy for enhancing the safety and cycling stability of anode materials lies in fabricating a solid electrolyte interphase (SEI) layer with excellent thermal stability and mechanical properties. For lithium metal batteries, using a lithiophilic matrix material can guide uniform lithium deposition and suppress dendrite formation. Atomic-level surface coating technology can significantly improve the thermal stability and safety of carbon-based and silicon-based anodes. Concurrently, smart anode materials can promptly terminate battery chemical reactions to prevent accidents.
Cathode materials:
Effectively reducing heat generation in batteries can be achieved by minimizing the gap between the theoretical and practical voltages or by enhancing cathode conductivity. Methods such as additive use, coating technology, and elemental substitution are conventional solutions widely applied to intercalation-type cathodes. Furthermore, coating the cathode with thermally sensitive materials can improve battery safety.
Electrolyte materials:
Additives containing triphenyl phosphate, silicon, and fluorine can enhance the thermal stability of liquid electrolytes. Additionally, highly active additives can suppress the formation of phosphorus pentafluoride (PF5) and hydrogen fluoride (HF) – reactive species generated from the decomposition of conventional LiPF6-based electrolytes, which inevitably damage the SEI and CEI layers. With deepening understanding of electrolytes, safe solid-state electrolytes hold broad application prospects.
Separator materials:
Employing materials with superior thermal stability, such as polyimide (PI) and polyphenylene sulfide (PPS), as separators, combined with modification treatments and surface coating technologies, can significantly enhance separator stability. Moreover, incorporating thermally sensitive materials can trigger a shutdown mechanism promptly in case of battery overheating, effectively preventing the occurrence of thermal runaway.
Conclusion
With the increasingly frequent use of lithium batteries, the safety issue of thermal runaway cannot be overlooked. Analyzing the heat sources of lithium batteries, addressing the process and causes of thermal runaway in lithium-ion batteries, and implementing various improvement methods to mitigate or prevent thermal runaway are effective means to enhance the safety of lithium batteries. This will provide reliable safety assurance for the widespread application of lithium batteries.
If you want to know about specific cases of electric vehicle fires, you can read
The Fire Risks Behind the Electric Vehicle Boom.
References
[1] Hydride-Ion Batteries: A Rising Star in the Battery World. (2025)
[2] Whittingham, M.S.: Lithium batteries and cathode materials. Chem. Rev. 104, 4271–4302 (2004).
[3] The Fire Risks Behind the Electric Vehicle Boom. (2025).
[4] Events with smoke, fire, extreme heat or explosion involving lithium batteries. Federal Aviation Administration Publishing. (2021).
[5] Zhang, T.S., Gao, C., Gao, Q., et al.: Status and development of electric vehicle integrated thermal management from BTM to HVAC. Appl. Therm. Eng. 88, 398–409 (2015).
[6] Viswanathan, V.V., Choi, D., Wang, D.H., et al.: Effect of entropy change of lithium intercalation in cathodes and anodes on Li-ion battery thermal management. J. Power Sources 195, 3720–3729 (2010).
[7] Kong L, Li Y, Feng W. Strategies to solve lithium battery thermal runaway: from mechanism to modification[J]. Electrochem. Energy Rev, 4, 633-679 (2021).











