Traditional hydrogen fuel cells primarily rely on the movement of protons (hydrogen positive ions), accompanied by charge transfer, to generate an electric current in an external circuit. Recently, the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences developed a novel core-shell structured hydride ion electrolyte and successfully constructed the world's first all-solid-state hydride-ion prototype battery using this electrolyte[1]. Previously, there were doubts about the practical feasibility of hydride-ion batteries. Hydride ions had even been used as a marketing gimmick, promoted under the banner of "antioxidant properties" as an additive in mineral water, a practice later banned entirely in Japan. Now, with the advent of the hydride-ion battery, hydrogen-based batteries have gained new possibilities.
1. Overview of hydrogen-based batteries
Based on the type of hydrogen ions involved, current systems can be classified into two categories: hydrogen fuel cells (proton-based) and hydride-ion batteries.
Basic working principle of hydrogen fuel cells
(taking proton exchange membrane fuel cell as an example, Figure 1):
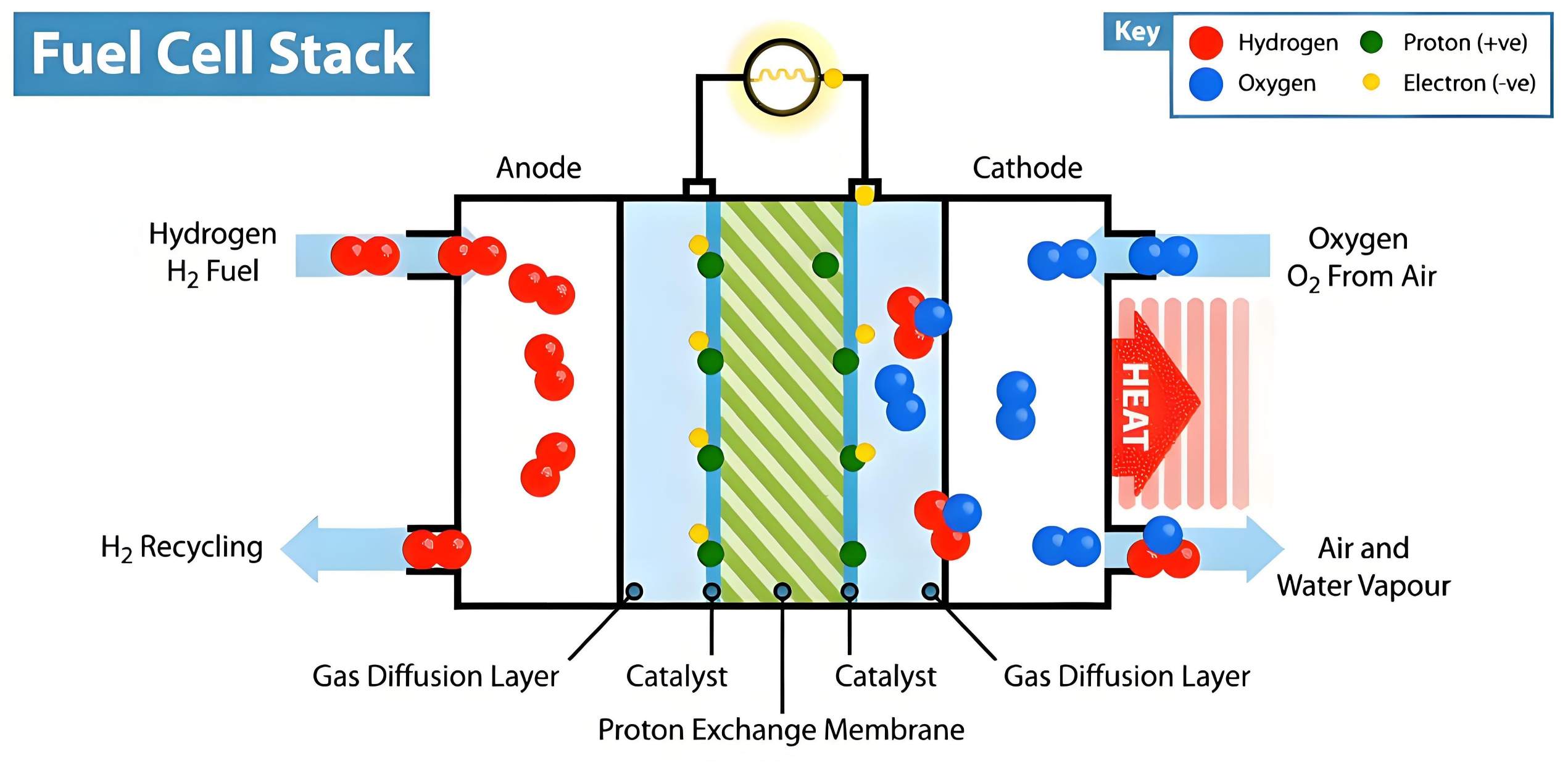
Figure 1 Working principle diagram of hydrogen fuel cell (proton exchange membrane fuel cell)
Hydrogen supply: Hydrogen is supplied to the anode (negative electrode).
Anodic reaction (oxidation reaction): At the anode catalyst (typically platinum), a hydrogen molecule is split into two protons (H+) and two electrons (e–).
Chemical reaction formula: H2 → 2H+ + 2e–
Ion transport: Protons (H+) are transported through the proton exchange membrane (PEM) in the center of the cell to reach the cathode (positive electrode). This membrane blocks the passage of electrons.
Electron transport: Electrons (e–) cannot pass through the proton exchange membrane and are therefore forced to flow through an external circuit to the cathode, thereby generating direct current (DC). This electric current can be used to power motors or supply energy to electronic devices.
Oxygen supply: Oxygen from the air is supplied to the cathode.
Cathodic reaction (reduction reaction): Protons (H+), electrons (e–), and oxygen (O2) combine at the cathode to form water (H2O).
Chemical reaction formula: O2 + 4H+ + 4e– → 2H2O
Byproduct removal: The water and heat generated by the reaction are removed as primary byproducts.
Basic working principle of hydride-ion battery
(Figure 2):
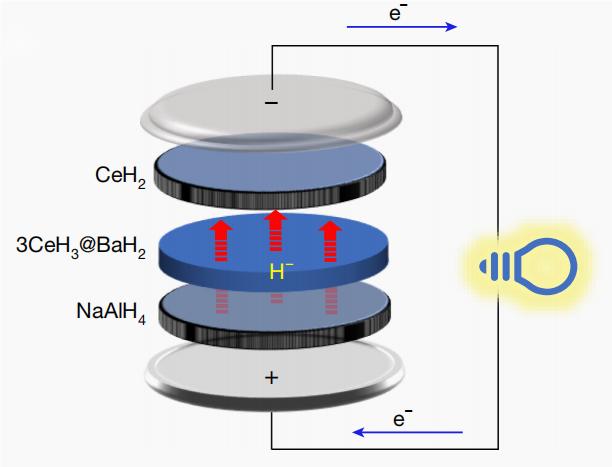
Figure 2 Working principle of hydride-ion battery
During discharge: NaAlH4 at the cathode directly releases hydride ions into the solid-state electrolyte 3CeH3@BaH2, while the released electrons flow from the cathode to the anode through the external circuit; at the anode, CeH2 absorbs the hydride ions and releases electrons.
During charging: CeH2 at the anode releases the hydride ions that were absorbed during the discharge process, while electrons flow from the anode to the cathode through the external circuit.
2. Unique characteristics of hydride-ion batteries
Hydride-ion batteries fundamentally operate through the movement of electrons between the positive and negative electrodes alongside the migration of hydride ions. Unlike proton-based hydrogen fuel cells, this process involves no combination of cations and anions and produces no byproducts. Differing from lithium-ion batteries, which use lithium ions as charge carriers, hydride-ion batteries utilize hydride ions as charge carriers, fundamentally avoiding the formation of metal dendrites.
The primary challenge for hydride-ion batteries lies in the conventional hydride electrolyte materials, which present several issues, such as compatibility problems with electrode materials, low hydride-ion conductivity, and electronic conductivity concerns. Even if some hydride materials can conduct hydride ions, they often fail to effectively suppress electron conduction. This results in electrons being unable to follow specific transfer paths within the material, leading to internal short circuits in the battery.
A team led by Professor Chen Ping, Professor Cao Hujun, and Associate Researcher Zhang Weijin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences innovatively designed a novel "core-shell structure" synthesized via a simple mechanical ball-milling method, involving the coating of CeH3 with BaH2. Compared to the well-crystallized CeH3, the conductivity of CeH3@BaH2 decreased from 3.0×10–2 S·cm–1 to 3.2×10–6 S·cm–1, a reduction of four orders of magnitude, as shown in Figure 3.
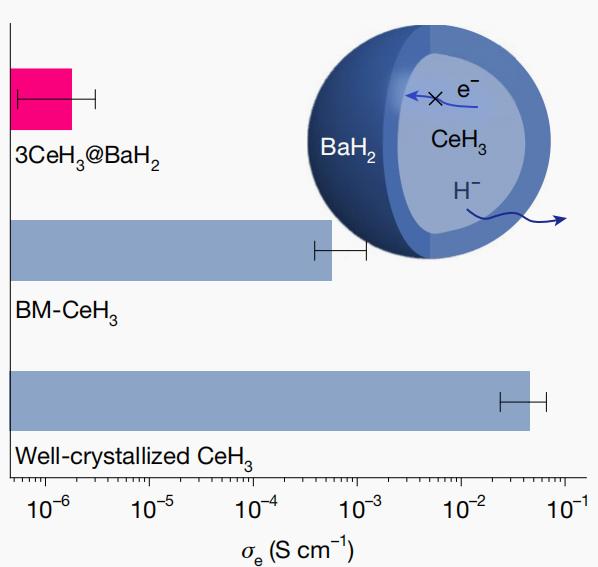
Figure 3 Room temperature electronic conductivity (σe) of well-crystallized CeH3, the BM-CeH3, and 3CeH3@BaH2 samples. Inset is the schematic of H– and electron transmission in the 3CeH3@BaH2
This improvement stems from structural deformation induced by mechanical processing and the unique coordination configuration formed at the interface (Ba and Ce share H-forming-distorted BaH5 and CeH6 coordination configurations that are different from their bulk phase structures, respectively). The electron-rich interface, combined with the wide bandgap (3.27 eV) of the BaH2 layer, inhibits electron transfer across the heterojunction. The BaH2 coating content significantly influences both electronic conductivity and hydride-ion conduction rate. While a high BaH2 content reduces both electronic conductivity and hydride-ion transport rate, an insufficient BaH2 content fails to effectively suppress electron conduction. The 3CeH3@BaH2 material with an optimized ratio demonstrates the best performance at room temperature. Electrochemical impedance spectroscopy (EIS) measurements confirm a hydride-ion conductivity exceeding 10–4 S·cm–1 and an ion transfer number greater than 0.99, successfully achieving the design targets, as shown in Figure 4.
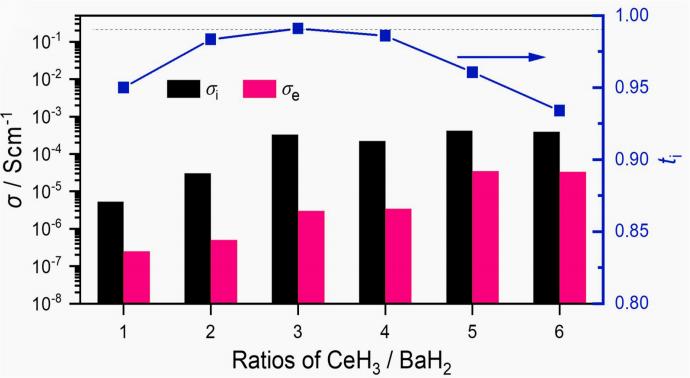
Figure 4 Ionic conductivity (σi), electronic conductivity (σe), and transfer number (ti) of nCeH3-BaH2 samples with different ratios at 20℃. The 3:1 ratio sample has a high ionic conductivity and the highest transfer number
In simple terms, this material design is analogous to constructing a highway for hydride ions while simultaneously creating barriers for electrons. The successful development of the CeH2|3CeH3@BaH2|NaAlH4 hydride-ion primary battery represents a major breakthrough in hydride-ion battery research. This hydride-ion battery operates effectively at room temperature without requiring elevated temperatures, while maintaining high hydride-ion conductivity—a key indicator of its strong potential for practical room-temperature applications.
3. Performance comparison
Hydrogen fuel cell (proton-based): The mainstream hydrogen fuel cell used in electric vehicles is the PEMFC (proton exchange membrane fuel cell). It operates within a temperature range of 60℃–80℃, offers quick start-up, high power density, and features a compact power generation module (Figure 5). Not being limited by the Carnot cycle, its actual comprehensive efficiency can reach 50%–60%. Coupled with hydrogen's inherent high energy density of 140 MJ/kg, refueling can be completed in as little as four minutes, enabling a medium-sized sedan or SUV to travel over 400 km. The output power of automotive fuel cells can reach up to 125 kW.

Figure 5 Internal structure of a hydrogen fuel cell vehicle (Honda CR-V e:FCEV)
The all-solid-state hydride-ion battery demonstrates a remarkable initial charge-discharge specific capacity of 984 mAh/g at room temperature. After 20 charge-discharge cycles, it maintains a specific capacity of 402 mAh/g, with a maximum voltage reaching 1.9V. The research team has successfully illuminated an LED lamp using this prototype, as shown in Figure 6.
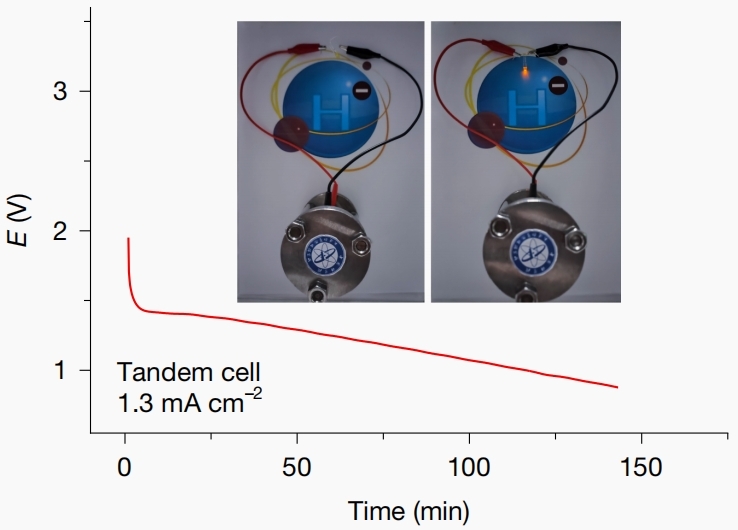
Figure 6 Discharge curve at a current of 1.3 mA cm–2 of the CeH2|3CeH3@BaH2|NaAlH4 tandem battery, and photos of the tandem battery in powering a yellow LED light
4. Market application
Hydrogen fuel cell (proton-based) is a power generation devices fundamentally, hydrogen fuel cells convert grid electricity into hydrogen energy, which is then directly transformed back into electrical power. They offer high thermal efficiency and deliver a superior user experience, with refueling times significantly faster than recharging. Their only emission is water, making them environmentally clean. Compared to traditional lithium-ion batteries that require extensive manufacturing and recycling processes, hydrogen fuel cells present a more sustainable solution. Several hydrogen vehicle models have entered production, such as the BMW iX5, though only a few (including the Hyundai Nexo, Toyota Mirai, and Honda CR-V e:FCEV) are publicly available for purchase. It is important to note that most hydrogen fuel cell electric vehicles are not powered solely by the fuel cell; they also incorporate high-performance batteries to enhance acceleration and high-speed cruising capability. Despite their advantages, hydrogen fuel cells face challenges including hydrogen storage and transportation, safety concerns, and the limited availability of refueling infrastructure. Furthermore, with rapid advancements in lithium battery technology—such as BYD's megawatt-level flash charging and Huawei's liquid-cooled supercharging technologies (Figure 7)—the future market competitiveness of hydrogen fuel cells remains uncertain and will depend on continued technological improvements.

Figure 7 BYD Megawatt-Level Flash Charging and Huawei Liquid-Cooled Supercharging
Hydride-ion solid-state batteries have transitioned from the conceptual stage to the laboratory stage. Compared to proton-based hydrogen fuel cells, they are capable of repeated charge and discharge cycles, making them truly rechargeable batteries. Although their energy density is significantly lower than that of traditional hydrogen fuel cells and their cycling performance still requires improvement, their energy density is considerably higher than that of lithium-ion batteries. Current testing equipment for hydride-ion solid-state batteries (the NEWARE solid-state mold and environmental test chambers, supporting environments from –70℃ to 150℃, and chamber volumes from 100L up to 1000L. Figure 8). However, for future market application, continuous improvements in cost, operating voltage, cycle life, and stability are still required.

Figure 8 Hydride-ion solid-state battery illuminating a bulb, NEWARE solid-state battery mold, and environmental test chamber
References
[1] Cui J, Zou R, Zhang W, et al. A room temperature rechargeable all-solid-state hydride ion battery[J]. Nature, 2025: 1-5.
Supplement
Some of the above materials are from the Internet. We are very sorry if there is any infringement! You can contact us for deletion!