What is true 5C fast charging?
In battery terminology, the "C-rate" measures the charge/discharge current relative to the battery's capacity:
1C = Fully charged in 1 hour
3C = Fully charged in 20 minutes
5C = Fully charged in approx. 12 minutes
The "5C fast charging" capability achieved in this research signals a potential reshaping of the charging experience across multiple sectors, from consumer electronics to electric vehicles. However, achieving such extreme charging speeds previously faced fundamental challenges: under extremely high current and voltage, the structure of battery materials rapidly degrades, leading to sharp performance decline and safety risks.
Core innovation: calculation-guided dual-stabilization strategy
The core of this breakthrough lies in the research team's development of a computationally guided "site-exchange" strategy. It innovatively reinforces both the "internal structure" and the "external surface" of the battery material simultaneously, thereby synergistically addressing the stability challenge of high-voltage LCO materials under fast charging.
Bulk reinforcement (sodium doping)
Sodium ions are strategically doped into the lithium cobalt oxide lattice, achieving dual enhancement:
Widened ion channels: Allow lithium ions to shuttle more rapidly, directly increasing charging speed.
Strengthened crystal framework: Enhances the material's structural robustness when operating at high voltages (>4.5V), preventing collapse from repeated expansion and contraction.
Surface protection (LMBO coating)
A robust amorphous LiₓMgᵧBO₂ (LMBO) coating is formed in situ on the cathode surface via magnesium-sodium ion exchange. This nanoscale layer (2-5 nm thick) acts as a multifunctional barrier:
Isolates chemical corrosion: Effectively prevents electrolyte erosion of the active material.
Reduces cobalt dissolution: Significantly suppresses harmful side reactions, preventing the dissolution and loss of beneficial components.
Maintains interface stability: Effectively inhibits interface degradation and harmful side reactions by stabilizing lattice oxygen.
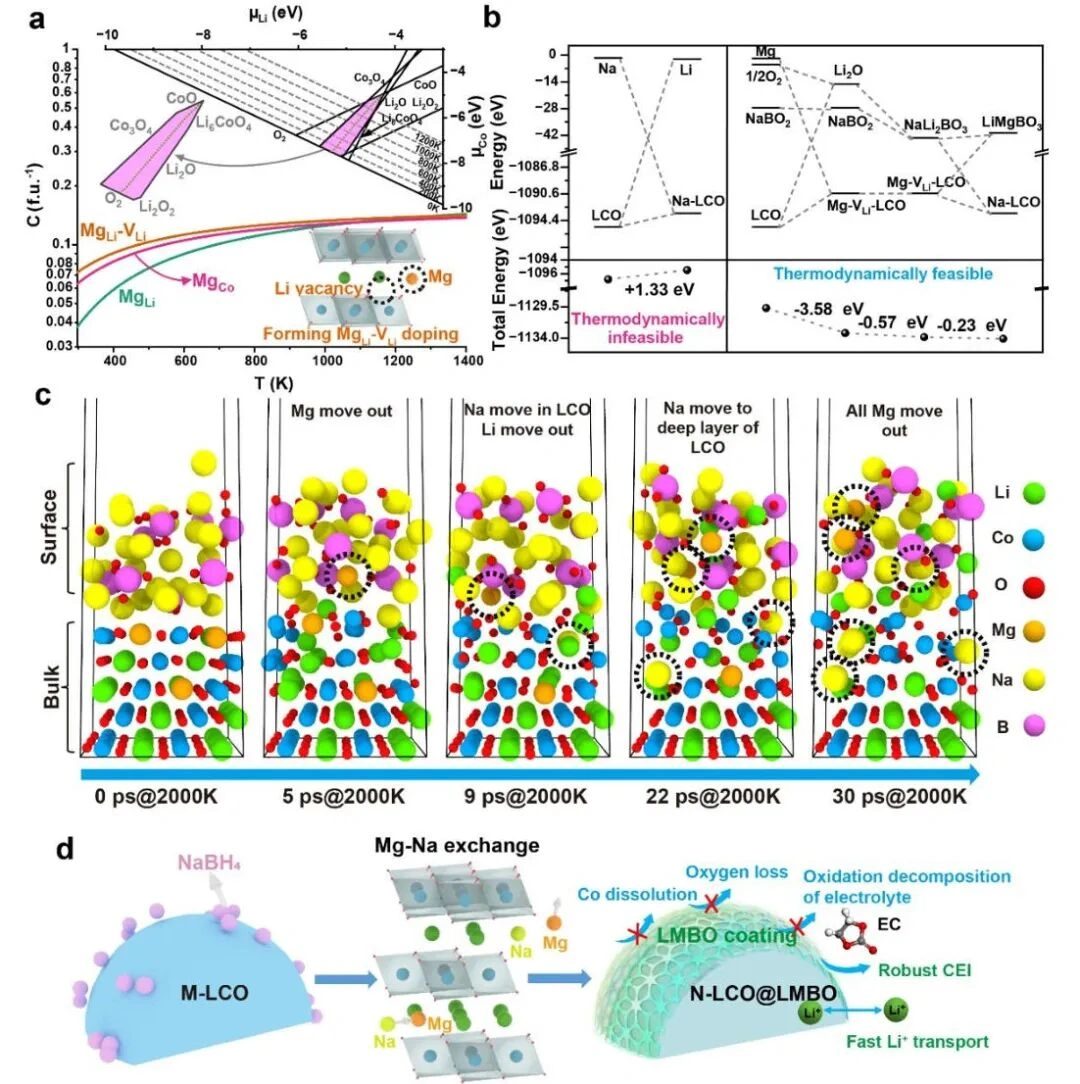
Figure 1. Core Mechanism of 5C Fast Charging: Calculation-Guided Mg-Na Ion Exchange and Dual-Stabilization Strategy ( Source: Adapted from Wang, T., et al. [1] )
Performance validation: setting a new benchmark for Fast-Charging batteries
The modified N-LCO@LMBO material demonstrates outstanding electrochemical performance under extreme conditions:
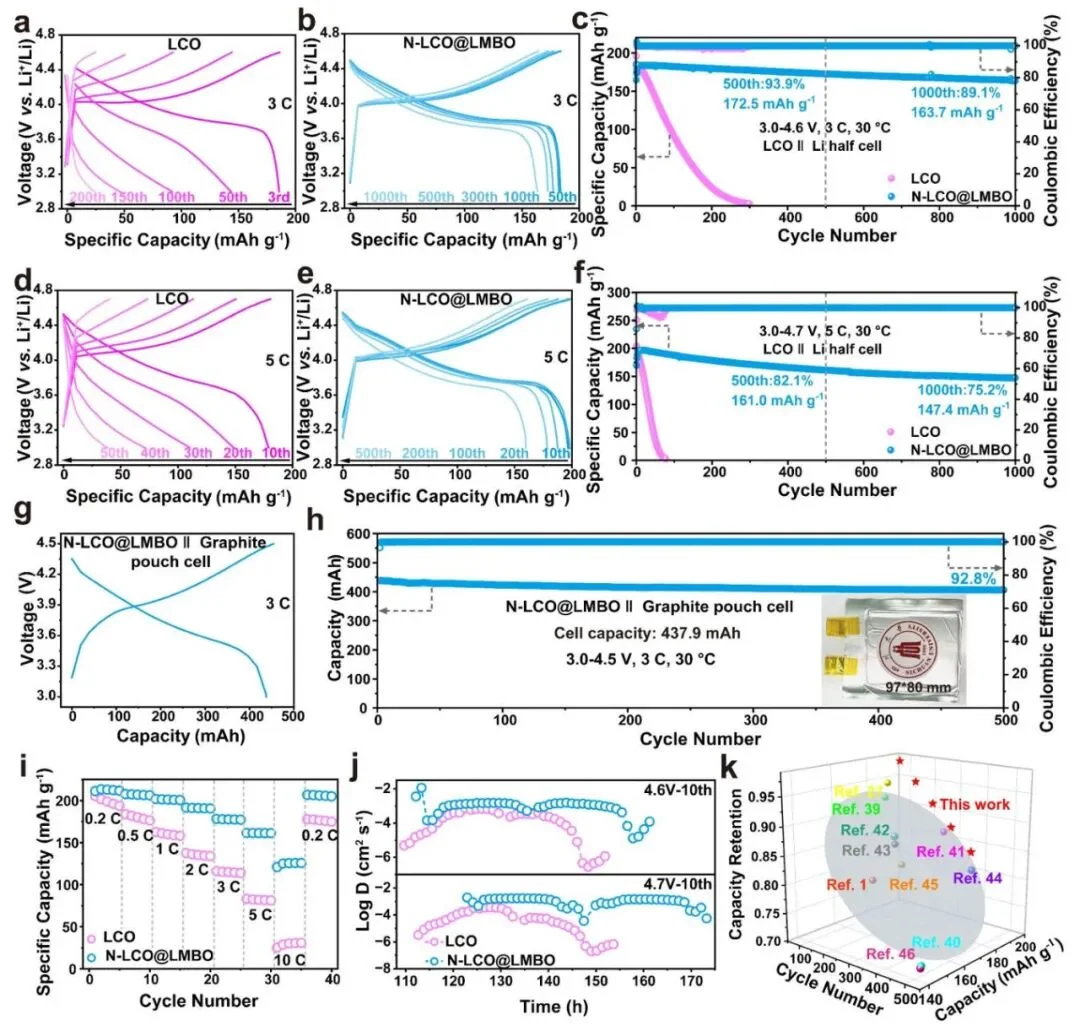
Figure 2. Performance Test Data: Cycle life and voltage curves of N-LCO@LMBO under 5C fast charging at 4.7V high voltage. (Source: Adapted from Wang, T., et al. [1])
| Test Condition | Performance Metric | Result | Industry Benchmark |
5C Fast Charge @ 4.7V Ultra-High Voltage | Capacity Retention after 500 Cycles | 82.1% | Traditional LCO usually <50% |
| 3C Fast Charge @ 4.6V High Voltage | Capacity after 1000 Cycles | 163.7 mAh g⁻¹ | Rapid decay within 200 cycles |
| Full Cell (Graphite Anode) @ 3C | Capacity Retention after 500 Cycles | 92.8% | Commercial batteries usually 70-80% |
Technical mechanism: why does it succeed?
In-depth analysis reveals the fundamental reasons for its success. Advanced characterization and computational modeling uncovered the underlying mechanisms:
Thermodynamic feasibility: Density functional theory (DFT) calculations confirm the magnesium-sodium exchange process is thermodynamically favorable, driving the spontaneous formation of the protective coating.
Synergistic stabilization effect: Sodium-doped bulk structure enhances intrinsic stability, while the LMBO coating provides external protection, creating a "reinforced concrete"-like structure for the cathode particles.
Interface engineering: The coating effectively suppresses harmful oxygen redox activity at high voltages (>4.5V), stabilizing lattice oxygen.
Commercial prospects and impact of 5C fast charging
This technological breakthrough in 5C fast charging has moved beyond the lab proof-of-concept stage, demonstrating clear commercialization potential:
Successful pouch Full-Cell tests: Pouch full cells assembled using N-LCO@LMBO cathodes and commercial graphite anodes show initial capacities exceeding 400mAh.
Scalable material process: The methods employed are compatible with commercial-grade raw materials, paving the way for large-scale production.
Potential safety enhancement: By suppressing side reactions, thermal risks associated with high-rate fast charging are reduced at the material level.
Its impact on the industry could be broad:
Consumer electronics: Future devices like smartphones and laptops could achieve full charges within the ten-minute range, with longer-lasting batteries.
Electric vehicles (EVs): Although this study is based on LCO material, the core design strategy of"synergistic bulk doping and surface coating stabilization"is universal. It provides a clear and validated technical paradigm for developing the fast-charging performance of mainstream EV cathode materials like NMC and LFP, offering significant guidance for solving the widespread fast-charging challenge in EVs.
Technology diffusion: The "synergistic stabilization" design philosophy provides a clear R&D paradigm for developing other, higher-performance battery material systems.
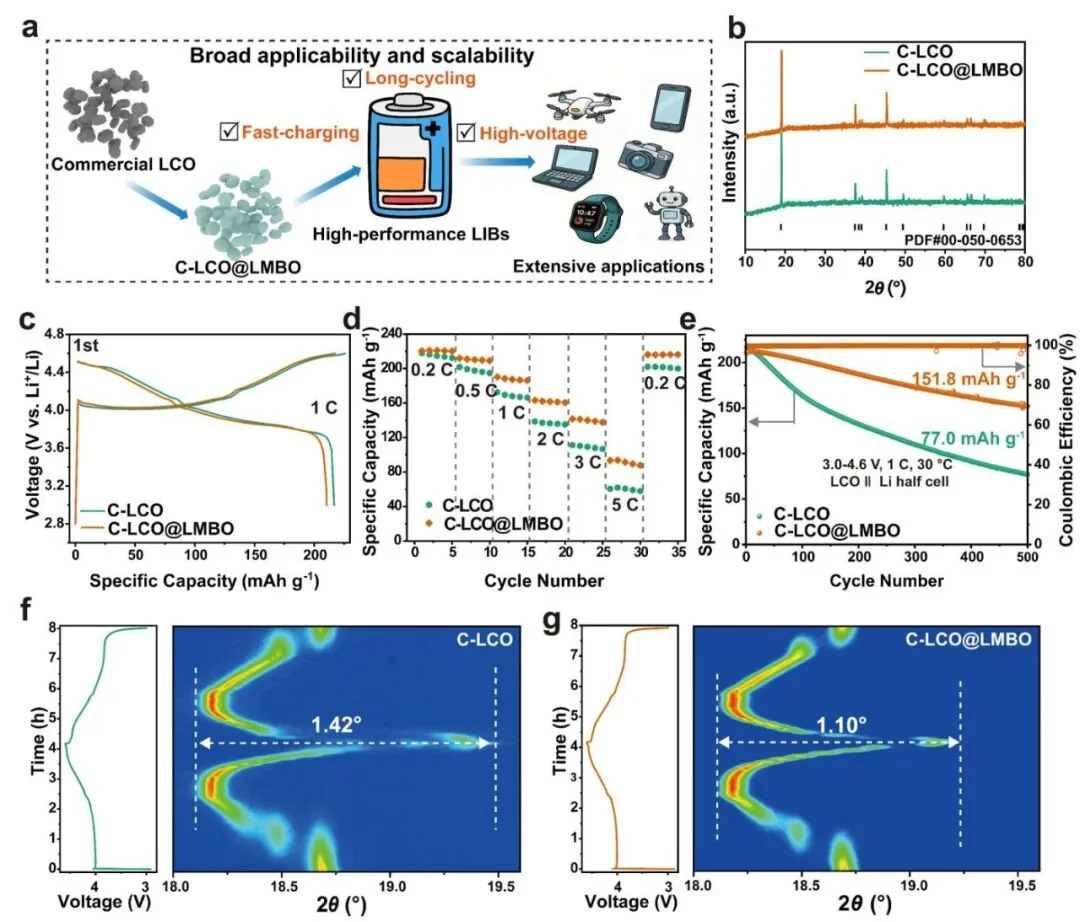
Figure 3. Commercial Validation: From Material Preparation and Pouch Cell Assembly to Multi-Field Applications (Source: Adapted from Wang, T., et al. [1])
Future outlook
The current research results illuminate a clear path forward for fast-charging battery technology. Future development will focus on:
Further optimizing materials and processes to continuously improve performance and reduce cost.
Accelerating collaboration with downstream battery manufacturers to complete engineering validation for scaled-up production.
Conducting more rigorous, long-term reliability assessments closer to real-world usage scenarios.
This breakthrough demonstrates that precise materials science and engineering design can effectively tackle core challenges in battery technology. As R&D deepens, the experience of safe and reliable ultra-fast charging is accelerating its transformation from a future blueprint into a tangible reality.
R&D Notes: how to verify 5C fast charging materials?
If you aim to precisely reproduce 5C fast charging test data found in top-tier journals, you need a testing system with millisecond-level response.
Research preferred for High-Rate: Learn about NEWARE CT-9000 High-Performance Battery Testing System (Supports 1000Hz sampling, ≤1ms response)
Cycle life verification: View the classic CT-4000 Series
Safety & temperature testing: High and Low-Temperature Environmental Chamber Solutions
References
[1] Wang, T., Meng, Y., Zhang, Y., et al. "Ion Exchange-Induced LixMgyBOz Coating Synergized with Reinforced Bulk Doping Enables Fast-Charging Long-Cycling High-Voltage LiCoO₂." Energy & Environmental Science(2025). DOI: 10.1039/d5ee04240b