Introduction
Coupling Cyclic Voltammetry (CV) with other testing techniques is a core strategy for gaining a more comprehensive understanding of battery behavior. Among the coupling techniques, the most widely used involves the complementary combination of conventional ex situ testing with CV testing. Conventional ex situ tests need to be performed sequentially; samples may need to be removed from the cell or there are intervals between tests. They can provide multi-dimensional, mutually verifying supplementary data, thereby constructing a more complete battery performance profile and enabling analysis of battery properties.
Conventional ex situ coupled testing
These are the most commonly used and easiest-to-implement combinations in both laboratory and industrial settings, typically performed sequentially on the same sample using different equipment.
Electrochemical impedance spectroscopy (EIS)
Coupling logic: CV (Qualitative/Kinetics) + EIS (Quantitative/Interface)
Synergistic Effect: CV can rapidly scan the active potential window and reaction reversibility of the battery. Based on this, performing EIS tests at a specific potential determined by CV (such as the open-circuit potential or a certain peak potential) allows for the quantitative analysis of the battery's ohmic resistance, charge transfer resistance, lithium-ion diffusion impedance, etc. The combination of both enables understanding "what reaction occurs" and also "where the resistance to the reaction comes from."
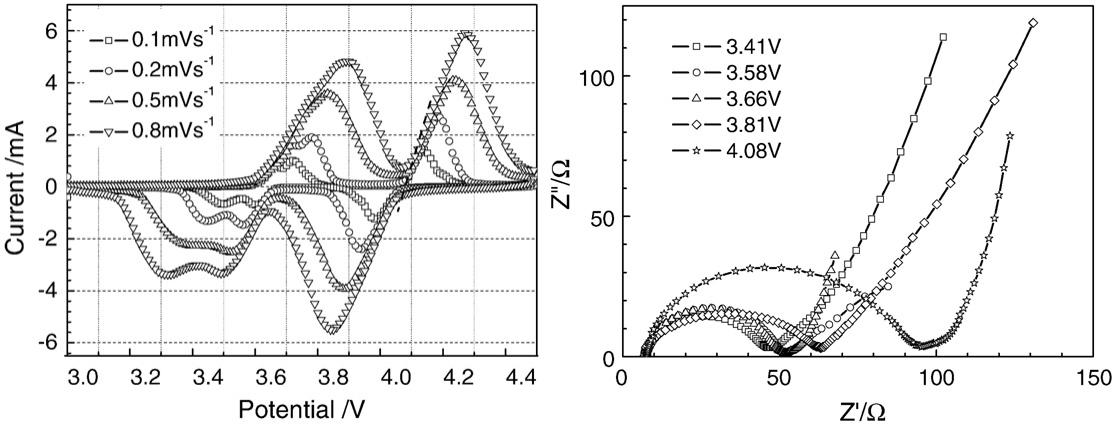
In a study measuring the chemical diffusion coefficient of lithium ions in Li3V2(PO4)3 material, CV and EIS provided independent yet mutually corroborating data pathways [1]. CV first qualitatively determined that the electrode reaction was controlled by the lithium-ion diffusion step by verifying a linear relationship between the peak current and the square root of the scan rate. Building on this, EIS was able to quantitatively calculate the chemical diffusion coefficient of lithium ions by analyzing its impedance data in the very low-frequency region. It is noteworthy that the diffusion coefficient values measured by CV and EIS methods respectively both fell within the range of 10-9 to 10-8 cm2/s, showing good consistency. This fully demonstrates the complementarity of CV in qualitative mechanistic judgment and EIS in quantitative calculation of key parameters.
>
Figure 1 Left: cyclic voltammetry curves of Li3V2(PO4)3 electrodes at different scan rates at 25℃. Right: Nyquist plots of Li3-xV2(PO4)3 measured at different open-circuit voltages [1]
Galvanostatic charge-discharge testing
Coupling logic: CV (Mechanism Investigation) + Charge-Discharge (Performance Verification)
Synergistic effect: Charge-discharge tests provide the most direct performance parameters, such as capacity, Coulombic efficiency, and cycle life. CV can be used to deeply analyze which specific electrochemical reactions (e.g., continuous growth of the SEI film, loss of active material) during the cycling process lead to capacity decay. For instance, by comparing the changes in the CV curves before and after cycling, the stability of the electrode material can be judged.
A study from Northeast Normal University focused on extending the cycle life of lithium metal batteries in carbonate-based electrolytes by designing a gradient solid electrolyte interphase (SEI) [2]. Charge-discharge tests intuitively demonstrated the improvement in battery cycling stability after applying the novel SEI. The role of CV testing here is to qualitatively or semi-quantitatively evaluate the influence of the SEI film on lithium-ion transport kinetics and the suppression of interfacial side reactions by observing parameters such as the sharpness of the lithium deposition/dissolution peaks and the peak potential separation. This helps explain the intrinsic mechanism behind the extended cycle life.
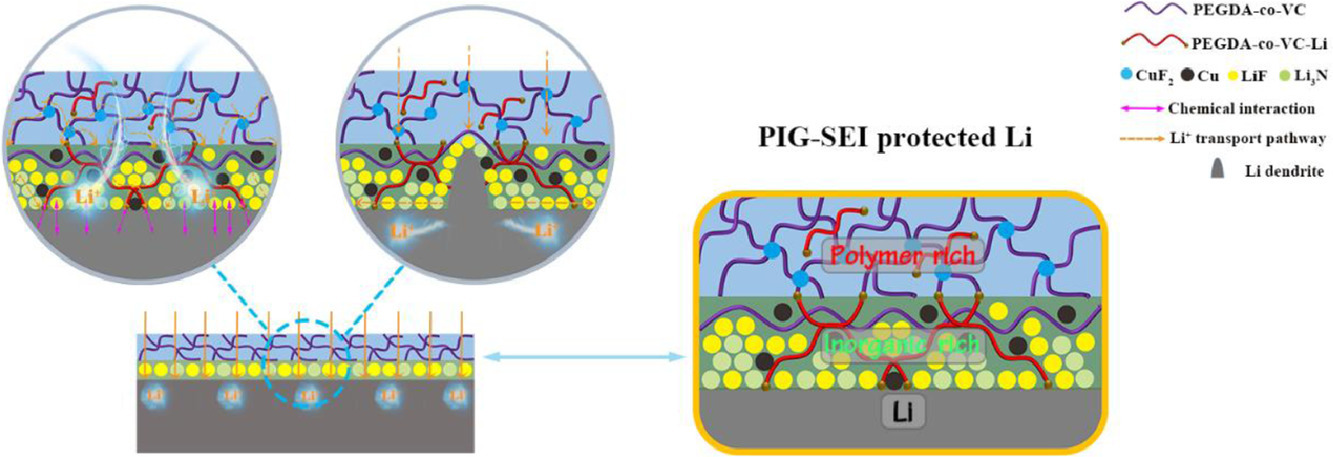
Figure 2 Polymer-inorganic gradient SEI (PIG-SEI) film formed in situ on the lithium metal surface [2]
Galvanostatic Intermittent Titration Technique (GITT)
Coupling logic: CV (Kinetic Trends) + GITT (Diffusion Coefficient Quantification)
Synergistic Effect: CV testing at different scan rates can qualitatively determine whether the reaction is controlled by surface capacitance or by diffusion. If the peak current is proportional to the scan rate, it usually indicates a surface-controlled process. If the peak current is proportional to the square root of the scan rate, it indicates a diffusion-controlled process. In this way, CV can quickly help us understand the general mechanism of the reaction and decide whether more precise GITT measurements are necessary to quantify the diffusion process. The GITT technique can precisely calculate the solid-state diffusion coefficient of lithium ions in the electrode material, providing solid quantitative data support for the qualitative conclusions from CV.
A study targeting NMC622 cathode material demonstrated the in-depth application of the GITT technique itself [3]. The research found that when the battery voltage exceeded 3.8 V, the electrode's relaxation behavior was abnormal and did not meet the theoretical prerequisites for traditional GITT analysis. By comparing with electrochemical impedance spectroscopy, the researchers pointed out that within this voltage range, the influence of liquid-phase diffusion (i.e., the migration of lithium ions in the electrolyte pores) becomes significant and may even dominate the entire process. Since GITT testing requires a very long time, using CV first to judge whether precise GITT measurement is necessary is a highly efficient testing strategy.
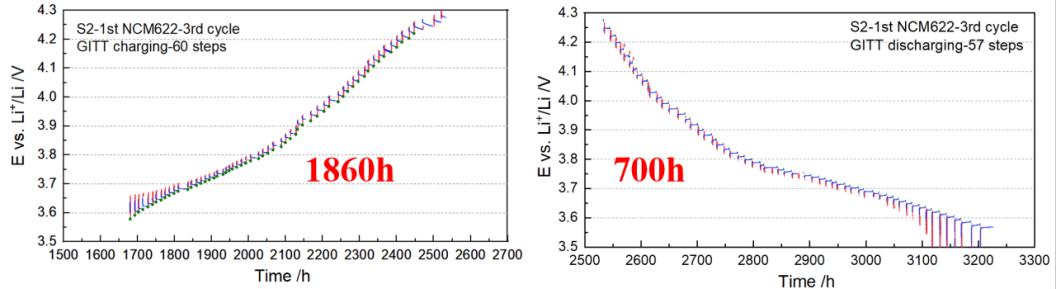
Figure 3 Second GITT test of the NMC622 battery (lasting 2570 hours, approximately 107 days)
Conclusion
The choice of which tests to combine with CV depends on your research objectives. For conventional performance characterization and failure analysis, CV + EIS + Galvanostatic Charge-Discharge forms the most classic and effective "triad" combination. If determining whether GITT testing is necessary, a preliminary assessment can be made using CV testing.
References
[1] Tang A, Wang X, Xu G, et al. Determination of the chemical diffusion coefficient of lithium in Li3V2(PO4)3[J]. Materials Letters, 2009, 63(16): 1439-1441.
[2] Lu W, Sun L, Zhao Y, et al. Elongating the cycle life of lithium metal batteries in carbonate electrolyte with gradient solid electrolyte interphase layer[J]. Energy Storage Materials, 2021, 34: 241-249.
[3] Abbas I, Tran H T, Tran T T N, et al. GITT Limitations and EIS Insights into Kinetics of NMC622[J]. Batteries, 2025, 11(6): 234.
[4] Moro G, Cristofori D, Bottari F, et al. Redesigning an electrochemical MIP sensor for PFOS: Practicalities and pitfalls[J]. Sensors, 2019, 19(20): 4433.