1. Introduction
Potentiostatic Intermittent Titration Technique (PITT) is an electrochemical method used to investigate the kinetic properties of electrode materials. By applying stepwise control of electrode potential, PITT measures the electrochemical response characteristics of electrode materials at different potentials, particularly examining ion diffusion behavior. Widely applied in electrochemical energy storage systems (e.g., Li-ion batteries, Mg-ion batteries), PITT provides critical information such as ion diffusion coefficients at varying potentials. This technique serves as a fundamental tool for characterizing diffusion and interfacial processes in electrode materials.
2. Principles and Methodology of PITT
2.1 Principles
The fundamental principle of PITT involves performing electrochemical titration on electrode materials at a series of constant potentials. At each applied potential, an electrochemical reaction occurs at the electrode, accompanied by gradual current decay. As time progresses, the current approaches zero, indicating that the system reaches electrochemical equilibrium – where the material's chemical potential equals the applied potential. By recording the current transient (current vs. time), the kinetic behavior of the material, particularly its diffusion properties, can be analyzed at each potential.
2.2 Experimental Procedure
Apply potential steps: Starting from an initial potential, the electrode potential is incrementally adjusted by a fixed step (e.g., 0.01 V or 0.05 V) to titrate the electrode material between successive potentials.
Record current decay: At each applied potential, the current decay profile (current vs. time) is recorded until equilibrium is reached.
- Repeat process: Steps 1-2 are repeated sequentially across all target potentials to obtain a complete titration curve (potential vs. current-time profiles).
2.3 PITT Data Analysis and Formulae
PITT data analysis is based on Fick's diffusion laws. When the electrode reaction rate is limited by ion diffusion, the diffusion coefficient DD is calculated from current decay using the following relationships:
Current-diffusion coefficient relationship:
Where:
i = current (A)
S = electrode/electrolyte contact area (cm²)
F = Faraday's constant (96,485 C/mol)
Cs = surface ion concentration at time tt (mol/cm³)
C0 = initial surface ion concentration (mol/cm³)
D = diffusion coefficient (cm²/s)
L = electrode thickness (cm)
Simplified solution for diffusion coefficient:
Diffusion coefficient calculation:
Experimental data is fitted to the equation, D is calculated from the slope (k) of the lnilni vs. t plot.
3. PITT Test Configuration
3.1 Instrumentation Overview
Based on PITT principles and calculation methods, diffusion coefficients (DD) can be determined after configuring appropriate test parameters. The Neware Multi-Channel Battery Test System (Fig. 1) is employed for PITT measurements in this setup.

Fig. 1 NEWARE Battery Testing System
The Neware multi-channel battery test system integrates numerous operational modes, including:
Charging modes—constant current (CC), constant voltage (CV), CC-CV, and constant power charging, with termination conditions (voltage, current, time, capacity, energy, -ΔV);
Discharging modes—CC, CV, CC-CV, constant power, and constant resistance discharging, with termination conditions (voltage, current, time, capacity, energy);
Pulse mode—charging (CC/constant power) and discharging (CC/constant power), featuring a minimum pulse width of 500 ms, support for up to 32 programmable pulses per step, charge-discharge switching within a single pulse step, and termination conditions (voltage/time);
DC internal resistance (DCIR) testing—enabling customized data-point selection for DCIR calculations;
Cycle testing range-1-65,535 cycles with 254 steps per cycle; (6) Nested cycling—supporting up to 3-tier nested loops. For additional functionalities, consult Neware technical personnel.
3.2 Testing Parameter Settings
Using a program starting from an open-circuit potential (OCP) of 1 V, with 0.1 V potential intervals, constant-voltage charging for 10 min per step, and cycling 6 times until reaching 1.5 V.
PITT Setup Procedure:
Charge the battery at constant voltage for 10 minutes.
Allow the cell to rest for 5 hours to reach equilibrium.
Repeat this sequence until the designated cutoff voltage is attained.
(Note: The discharge procedure follows an analogous process to charging.)
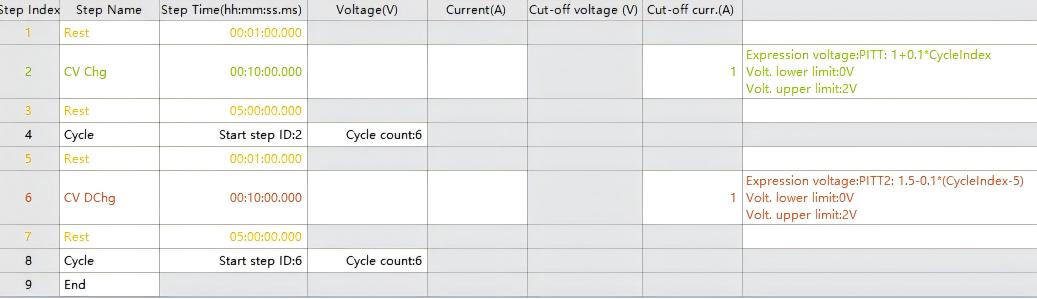
Fig. 2 PITT Test Protocol
Detailed Program Setup Procedure
Step 1. Configure the charge/discharge cycling and resting procedures as follows:
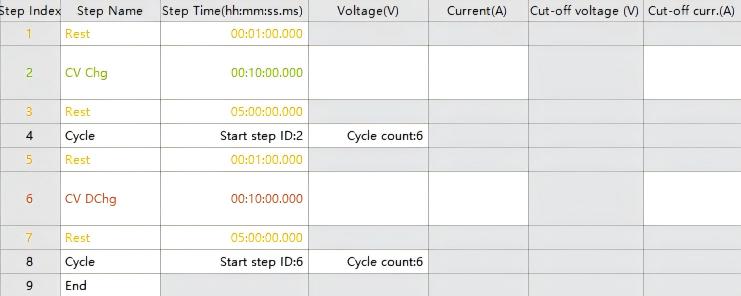
Fig. 3 Cycling and Resting Program Configuration
Step 2. Edit the voltage expression according to the method shown in the diagram below:
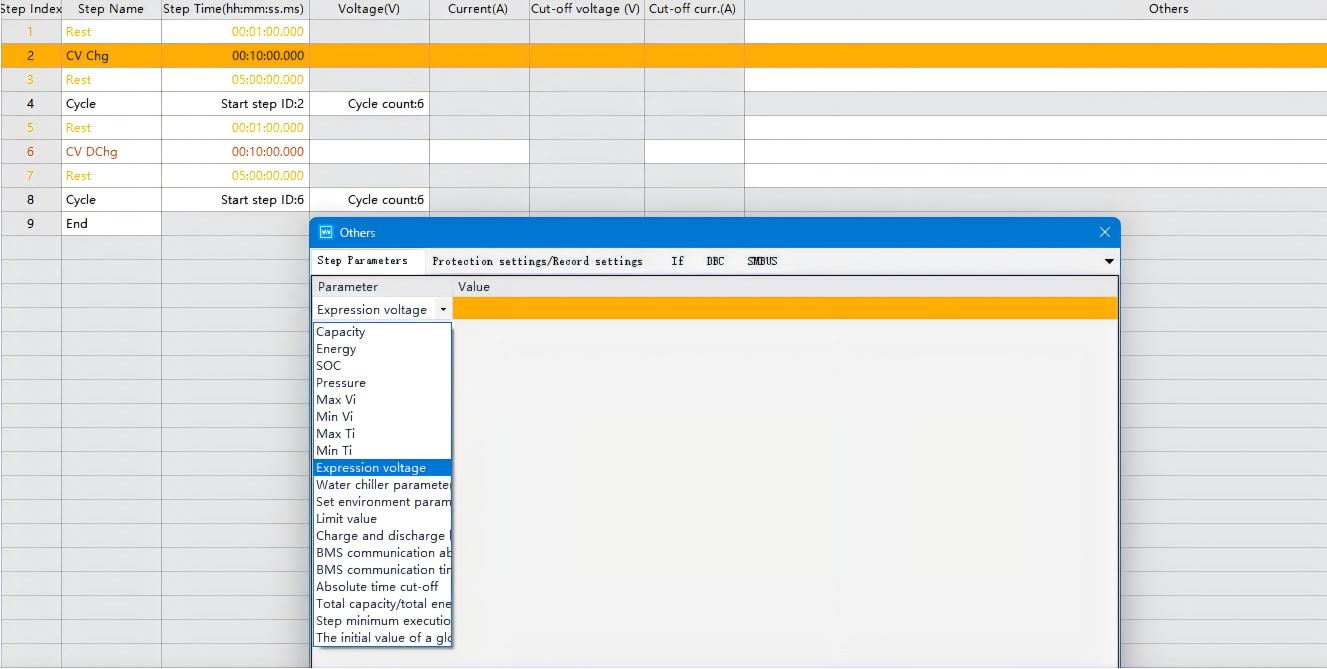
Fig. 4 Charging Process Voltage Expression Program Configuration
The edited voltage expression is shown in the diagram below:
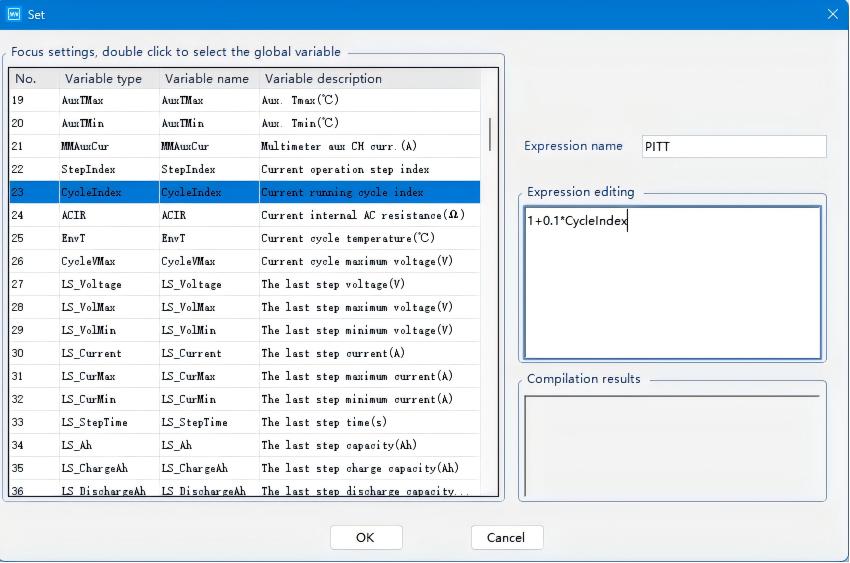
Fig. 5 Resulting Voltage Expression Program Configuration for the Charging Process
Then proceed to configure voltage protection parameters under the Protection Settings/Recording Conditions:
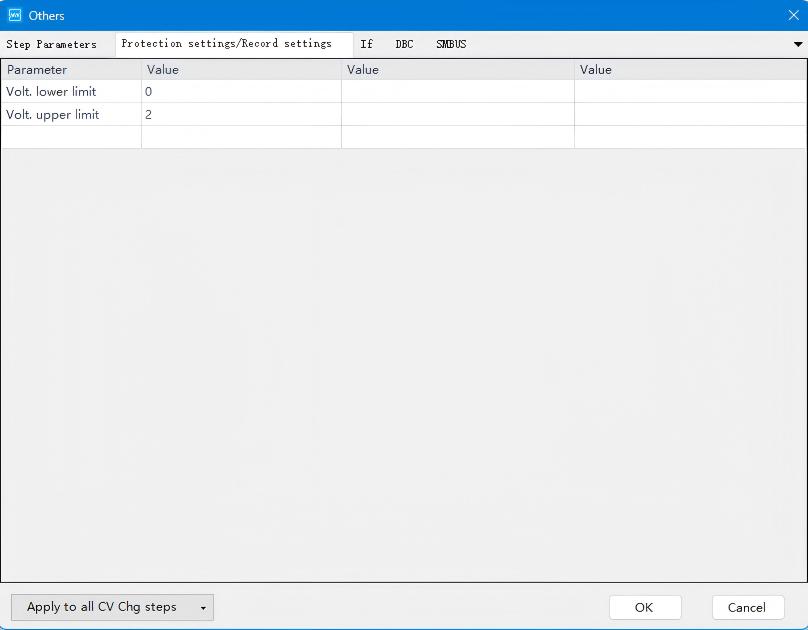
Fig. 6 Voltage Protection Configuration
The discharge procedure configuration mirrors the charging process, with the resulting voltage expression settings shown in the diagram below:
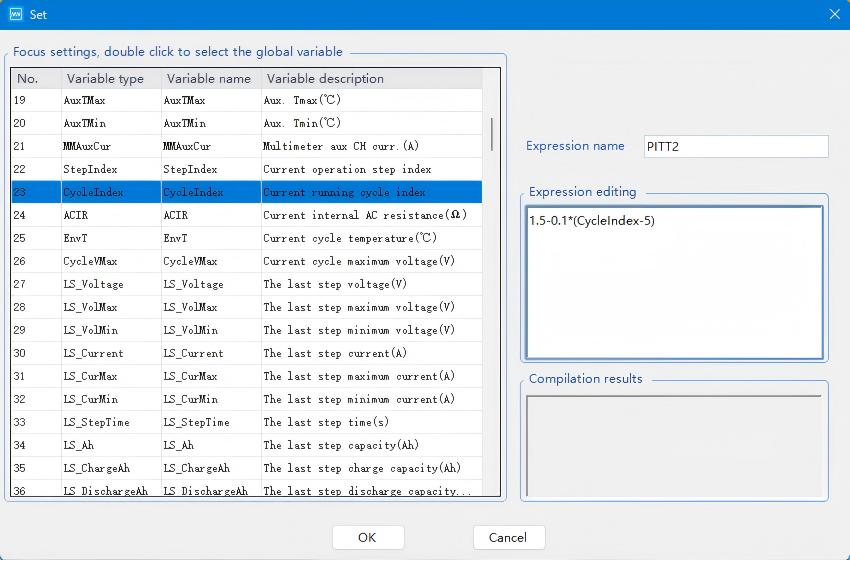
Fig. 7 Resulting Voltage Expression Program Configuration for the Charging Process
The PITT test results for commercial lithium-ion batteries are shown in the figure below:
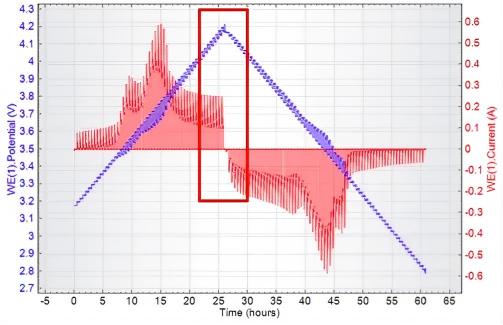
Fig. 8 Relationship Between Potential (Blue), Current (Red), and Time in PITT Testing
When the region in Figure 8 is magnified, the detailed view shown below is obtained:
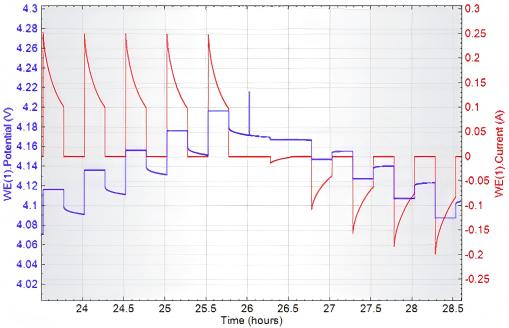
Fig. 9 Relationship Between Potential (Blue), Current (Red), and Time in PITT Testing
Further converting the current i to logarithmic form ln(i) yields the following plot:
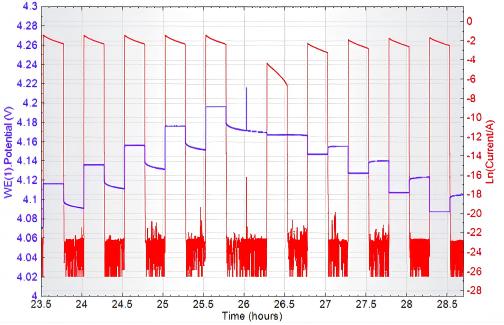
Fig. 10 Potential, ln(i), and Time t Relationships
For a specific segment of the ln(i) data, the diffusion coefficient D can be determined from the approximately linear region.
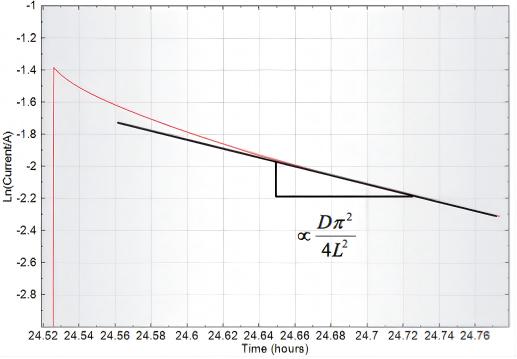
Fig. 11 A Plot of ln(i) Data
4. Advantages and Applications of PITT
In summary, as a non-destructive intermittent potential control technique, PITT enables precise determination of diffusion coefficients in electrode materials at various state-of-charge/discharge states, offering the following advantages:
Acquisition of detailed kinetic information: Particularly suitable for investigating ionic diffusion characteristics and interfacial reaction kinetics in materials.
Broad applicability in battery material research: Extensively employed in energy storage systems including Li-ion, Na-ion, and Ca-ion batteries, facilitating fundamental understanding of material performance and ion transport behavior during battery operation.
High efficiency and operational simplicity: Compared to constant-current diffusion measurement methods, PITT achieves accurate kinetic parameter extraction within significantly reduced experimental timeframes.
The data acquired through PITT not only provides fundamental insights into material kinetic behavior, but also guides the optimization of electrode architecture and design, thereby enhancing overall cell performance.