1. Introduction
The occurrence of low voltage failures during the lithium battery formation process is a relatively common yet critical issue, typically indicating abnormalities during the initial charging and activation of the cell.
2. Analysis of Main Causes for Low Voltage Failures
Firstly, raw material defects or incoming material quality issues are among the root causes. Defects in the anode material, such as active material imperfections, poor conductivity, or uneven coating, can directly hinder lithium intercalation and consume excessive lithium ions. Abnormal porosity, uneven thickness, or poor wettability of the separator can affect electrolyte infiltration and ion transport. Insufficient electrolyte volume, improper composition, or the presence of impurities can trigger side reactions and deplete active lithium. Defects in the cathode material, excessively high residual alkali, as well as contamination or oxidation of the current collectors, are also significant concerns. Furthermore, uneven dispersion or subpar performance of auxiliary materials like binders and conductive agents can create hidden risks.
Secondly, defects arising during the cell manufacturing process are equally critical. Issues in stacking/winding, such as electrode misalignment or irregular winding, can lead to localized lithium plating or short circuits. Poor welding, including false or cold soldering, results in high contact resistance. Inadequate electrolyte filling, insufficient wetting, or poor environmental control can create "dry areas" within the cell, preventing effective ion conduction. Poor sealing during encapsulation may cause electrolyte leakage or allow ingress of contaminants. Inadequate cleanliness control can introduce metallic foreign particles and dust, leading to micro-shorts.
Improper formation process settings can also directly cause low voltage. Excessively high charging currents not only increase polarization but can also lead to unstable SEI film formation or even induce lithium plating. Setting the charge cutoff voltage too low or insufficient constant voltage time can prevent complete lithium ion intercalation. Temperature control that is too high or too low affects ionic conductivity and the extent of side reactions. Insufficient rest time or an ill-designed process flow can hinder proper electrolyte wetting and reaction equilibrium.
Failures in the formation equipment or testing systems themselves cannot be overlooked. Hardware or software issues, such as abnormal formation channel operation, poor fixture contact, voltage detection errors, or programming mistakes, can all cause false low voltage readings.
3. Impact of Low Voltage Failures on Cells
Once a low voltage failure occurs, the cell often suffers irreversible damage, severely impacting its performance and safety. Its capacity will experience a significant and irreversible decline, primarily due to the irreversible consumption of active lithium and the failure of active materials to fully activate. Cycle life will also be severely shortened, attributable to continuous SEI film growth, worsening micro-short points, and structural damage to the anode. Concurrently, the cell's self-discharge rate will increase markedly, and internal resistance will rise, not only affecting rate capability but also leading to increased heat generation during operation.
More critically, safety risks escalate dramatically. Cells with low voltage failures have a high risk of lithium plating; lithium dendrites could pierce the separator, causing severe internal short circuits or even thermal runaway. Existing micro-short points may expand during subsequent use, and increased gas generation could lead to cell swelling or rupture. Furthermore, if such cells are incorporated into a battery pack, they become the "weak link," severely impacting the pack's consistency, overall lifespan, and safety.
Low voltage failures during the formation stage are a crucial indicator of serious cell defects or process abnormalities. The root cause lies in the irreversible consumption of active lithium (due to side reactions, lithium plating, micro-short consumption) and/or hindered lithium ion transport/intercalation paths (poor wetting, poor contact, material defects). The consequences are extremely severe, leading to permanent capacity loss, drastically reduced cycle life, high self-discharge, high internal resistance, and significantly elevated safety risks.
4. Summary
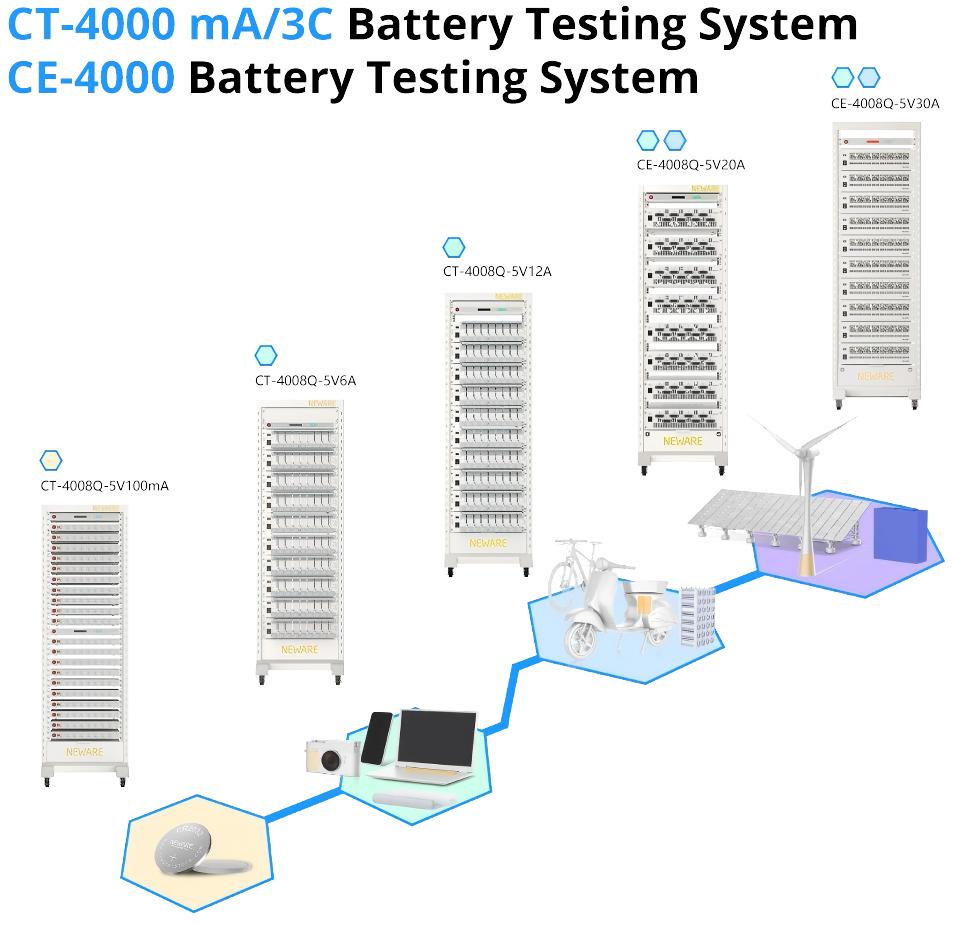
Therefore, monitoring and analyzing low voltage failures during formation is a vital part of quality control in lithium battery production, directly impacting the performance and safety of the final product. When the detection system lacks sufficient precision, these initial, subtle voltage anomaly signals can easily be missed, akin to planting a hidden "time bomb" within the battery. Selecting high-performance, high-precision battery test equipment is no longer merely an auxiliary production step but a crucial line of defense. The NEWARE CT/CE-4000 Series Battery Testing System, designed for high-precision battery testing, supports research across various applications including 3C single cells, solid-state batteries, and battery materials. Featuring a four-range design, its measurement accuracy reaches up to ±0.05% f.s., meeting precise testing requirements from microampere (μA) to milliampere (mA) levels. It offers a portable battery test solution with a Type-C power interface and adds CV and EIS testing capabilities to meet electrochemical testing needs. Beyond standard charge-discharge testing functions, it integrates various test modes such as EIS, DCIR, CV, and pulse simulation, fulfilling comprehensive testing requirements.