What are the Charge/Discharge Cutoff Voltages for Lithium-Ion Batteries?
The charge/discharge cutoff voltages are the upper and lower voltage limits set during battery operation to prevent damage, ensure safety, and extend cycle life.
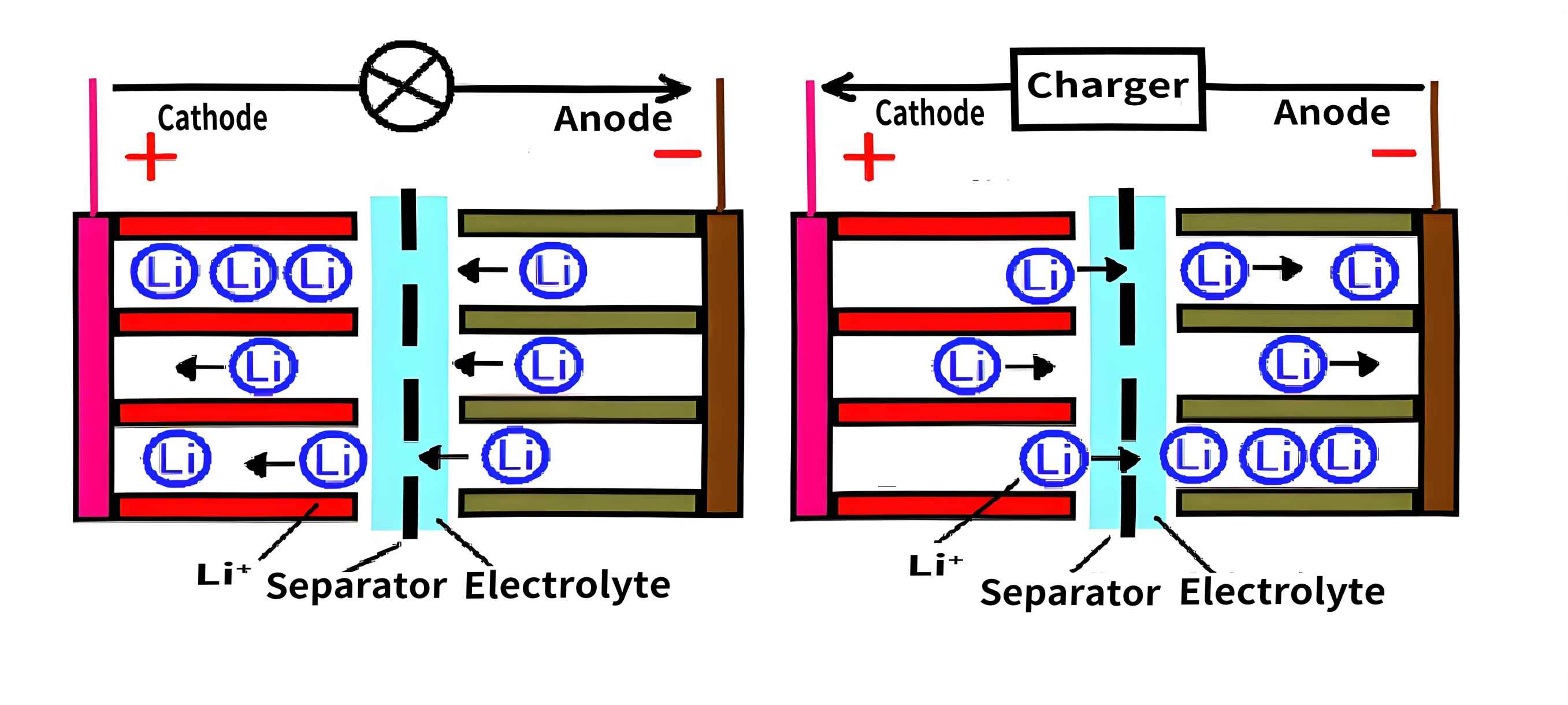
Lithium-Ion Battery Charging/Discharging Schematic Diagram
Charge Cutoff Voltage
Definition
The maximum voltage allowed during charging. Upon reaching this voltage, charging must stop or switch to trickle charging/termination.
Purposes
Prevent overcharging: Continued charging beyond this limit is termed overcharging.
Protect cathode materials: Excessive voltage causes irreversible structural changes (e.g., lattice collapse, oxygen release) in cathodes (e.g., LiCoO₂, NCM), leading to permanent capacity fade or thermal runaway (fire/explosion).
Protect anode materials: High voltage causes excessive Li⁺ intercalation into the anode (typically graphite), inducing lithium plating where metallic lithium deposits on the anode surface. This forms lithium dendrites that may pierce the separator, causing internal short circuits and severe safety hazards. Lithium plating also consumes active Li⁺ ions, reducing capacity.
Prevent electrolyte decomposition: High voltage accelerates electrolyte oxidation, generating gases (swelling) and byproducts, increasing internal resistance and degrading performance/safety.
Typical Values
3.6V to 4.5V, depending on cathode chemistry:
LiCoO₂ (LCO): ~4.2V
NCM/NCA: ~4.2V or 4.35V (high-voltage variants)
LiFePO₄ (LFP): ~3.6V–3.65V
LiMn₂O₄ (LMO): ~4.2V
Discharge Cutoff Voltage
Definition
The minimum voltage allowed during discharge. Discharge must terminate when this voltage is reached.
Purposes
Prevent over-discharge: Discharging below this limit is termed over-discharge.
Protect anode current collector: During deep discharge, excessive Li⁺ deintercalation from graphite raises the anode potential. At very low voltages (typically <2.5V–3.0V), the copper current collector oxidizes and dissolves. Dissolved Cu⁺ ions can migrate to the cathode or deposit on the separator during subsequent charging, causing micro-shorts and severe degradation.
Protect cathode materials: Certain cathodes undergo structural damage during deep discharge.
Prevent irreversible capacity loss: Over-discharge causes irreversible changes to active materials, permanently reducing capacity.
Avoid cell failure: Severe over-discharge renders the battery irrecoverable.
Typical Values
2.5V to 3.0V (less variable than charge cutoffs), commonly set at 2.5V, 2.8V, or 3.0V depending on cell chemistry.
Setting Voltage Limits in Experiments
When configuring voltage ranges for testing instruments, strict adherence to these principles is required. Limits must be determined based on the specific cathode/anode materials to prevent overcharging/over-discharging, ensuring experimental validity and safety.
Example: Cycling Parameters for Li-Ion Coin Cells
Select constant current cycle mode in NEWARE BTS software.
Test assembled cells at 0.1 A/g within the appropriate voltage range (e.g., 1 mA current for 10 mg active material).
Cathode testing: Set voltage range according to material (e.g., 2.0–4.2V for LFP//Li cells). Determine cutoffs via literature or polarization curves.
Anode testing: Sequence: Rest → Constant current discharge → Constant current charge.
Parameters: Current = 1 mA, discharge cutoff = 0.01V, charge cutoff = 2.0V, cycles = 2000.
Note: Voltage ranges are for reference only; actual ranges depend on specific materials.
Key Determinants for Setting Charge/Discharge Cutoff Voltages in Li-Ion Batteries
The cutoff voltages are primarily governed by the thermodynamic stability (chemical stability) of electrode materials. For cathode materials (e.g., LiCoO₂, NCM/NCA, LiFePO₄, LiMn₂O₄), excessively high charging voltages can trigger irreversible structural phase transitions, lattice oxygen release, dissolution of transition metal ions, or violent oxidative side reactions with the electrolyte. These phenomena accelerate capacity fade, increase internal resistance, and may induce thermal runaway.
In anode materials (primarily graphite), excessively low discharge voltages (corresponding to high anode potentials) cause over-delithiation, leading to structural collapse (exfoliation/pulverization). Crucially, when the anode potential drops below the reduction potential of the electrolyte, continuous reductive decomposition occurs at the anode surface. This thickens the solid electrolyte interphase (SEI) or causes metallic lithium plating (lithium dendrites). Dendrites may penetrate the separator, causing internal short circuits and severe safety hazards.
The electrolyte (typically lithium salt in organic carbonate mixtures) possesses a stable electrochemical window. Exceeding its upper voltage limit induces oxidative decomposition at the cathode, while operating below its lower limit causes reductive decomposition at the anode. These parasitic reactions consume active lithium and electrolyte, generate gases, degrade the SEI, and impair performance.
Cycle life is critically influenced by voltage management:
Deep cycling (using wider voltage windows) increases single-cycle capacity but accelerates electrode degradation and side reactions, drastically shortening battery lifespan.
Optimizing cutoff voltages to avoid extreme potentials is essential for longevity. Slightly raising the discharge cutoff (e.g., 2.5V → 2.8V/3.0V) or lowering the charge cutoff (e.g., 4.2V → 4.1V) sacrifices marginal capacity but significantly extends cycle life.
Energy density and capacity directly correlate with the voltage window. Wider windows enable greater lithium-ion extraction, yielding higher capacity and energy density.
Safety considerations are paramount:
Overcharging (exceeding charge cutoff) poses extreme risks: cathode decomposition releases oxygen, electrolyte oxidation generates heat, and lithium plating forms dendrites. These may culminate in thermal runaway, fire, or explosion.
Over-discharging (below discharge cutoff), while less immediately hazardous, dissolves the copper current collector at low potentials. During subsequent charging, dissolved copper ions may plate as dendrites, creating short-circuit risks and permanently degrading performance.
Strict adherence to cutoff voltages constitutes the primary and most critical safety barrier.
Application-specific requirements dictate voltage strategies:
Consumer electronics (phones/laptops): Prioritize energy density, often employing wider windows (e.g., 3.0V–4.2V/4.35V) with moderate lifespan expectations.
Electric vehicles: Balance energy density, power, longevity, and safety. Conservative windows are standard (e.g., NCM: 3.0V–4.2V; LFP: 2.5V–3.65V) with stringent BMS controls.
Energy storage systems: Emphasize ultra-long life and cost-efficiency. Narrow windows are typical (e.g., LFP at 3.0V–3.4V, utilizing ~40% SOC) to maximize cycle life.
Precise setting and rigorous control of cutoff voltages form the cornerstone of lithium-ion battery safety, cycle life optimization, and energy density-reliability balance. This principle underpins critical activities: developing novel electrode materials, optimizing electrolytes, designing BMS strategies, and validating extreme-condition performance—all requiring precise, reproducible testing within strictly defined voltage/temperature windows.
In battery R&D, manufacturing, and quality control, simulating charge-discharge behaviors across temperatures (high-temperature accelerated aging, low-temperature validation, room-temperature cycling) is indispensable. Accurately capturing battery states at voltage cutoffs—capacity, internal resistance, temperature rise, coulombic efficiency—reveals material boundaries, optimizes processes, verifies BMS logic, predicts lifespan, and assesses safety risks. Temperature, as a key variable affecting electrode kinetics, interface stability, electrolyte transport, and side reactions, synergizes with voltage management to define holistic battery performance and safety margins.