Introduction
Cyclic Voltammetry (CV) is an important and widely used electrochemical analysis technique. It plays an irreplaceable role in analyzing various electrochemical properties of materials during charge-discharge cycles, such as phase transformations, electrode reaction rates, rate-controlling steps, and reaction kinetics. By applying a specific voltage and measuring the changes in current and voltage, the electrochemical behavior curve of the material can be obtained. This clarifies the fundamental processes of the electrode reaction and the key factors influencing its progression, enabling effective deliberate control over the direction and rate of the electrode reaction. Consequently, it provides reliable theoretical guidance for both production and scientific research.
Multi-sweep-rate CV refers to CV measurements obtained at different scan rates. The significance of CV curves at varying scan rates lies in the ability to assess the reversibility of the electrode reaction based on the curve shape, as well as the possibility of intermediates, phase-boundary adsorption, or new phase formation, and the nature of coupled chemical reactions. It is extensively applied in fields such as battery materials and catalysts.
Principles and Methodology of Multi-Sweep-Rate CV Testing
Cyclic Voltammetry (CV) is a form of reversed double potential-step chronoamperometry. After a certain time interval, the potential reverses direction and scans toward positive potentials. As the potential continuously increases, the tendency for oxidation reactions intensifies, generating an oxidation current. For highly reversible reactions, the reverse-direction current exhibits a similar shape to the forward-direction current.
CV investigates the kinetics and mechanisms of electrochemical reactions by applying a linearly varying potential (voltage) to the electrode surface and monitoring the corresponding current response. This method involves controlling the electrode potential and recording the current flowing through the electrode during the potential variation, resulting in a current-potential (i-E) plot.
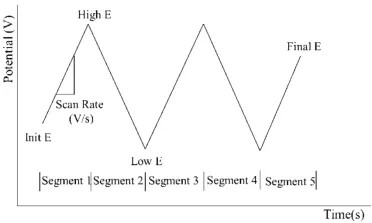
Method Principle:
This technique applies a linearly changing voltage (constant-slope voltage ramp) to an electrode. The scanning range is typically confined within ±3 V of the rest potential, where most electrode reactions occur, and generally does not exceed ±5 V.
Scanning Equations:
In cyclic voltammetry, the starting scan potential is expressed as:
E = Ei – vt (1)
where:
Ei: Initial potential
t: Time
v: Potential change rate or scan rate
The reverse scan is defined by:
E = Ei + v′t (2)
where v′ often equals v.
Current Response Mechanism:
When combined with an appropriate form of the Nernst equation, these expressions yield a formula describing particle flux at the electrode surface. This formula can be solved through stepwise numerical integration.
As the applied voltage approaches the reversible potential of the electrode process, a small initial current is observed. This current then undergoes rapid acceleration due to enhanced reaction kinetics. However, as reactants become depleted near the electrode surface, the current reaches a finite limiting value at potentials slightly exceeding the standard potential. This transition marks the onset of diffusion-limited behavior, where reactant depletion establishes a concentration gradient extending into the bulk solution. As the diffusion layer progressively thickens, the mass transfer rate via diffusion at the electrode interface correspondingly decreases. Consequently, after attaining its maximum value (peak current), the current exhibits a characteristic decline despite further voltage changes, demonstrating the dominance of diffusion-controlled mass transport over electrochemical kinetics.
The peak current for reversible reduction is defined as:
(3)
ip: Peak current
n: Electron transfer number
F: Faraday's constant (96,485 C/mol)
D: Diffusion coefficient of reactant
C0: Concentration of oxidized-state reactant
A: Electrode area
Analytical Capabilities of CV: CV provides both qualitative and quantitative information about electrode processes. For diffusion-controlled reversible reactions, this manifests as:
(4)
Key Characteristics:
Reversible Systems:
This peak potential separation (ΔEp) is independent of scan rate.
Non-Diffusion-Controlled Reactions (e.g., insoluble film oxidation):
For reversible oxidation of electrodeposited insoluble films, if the process is not diffusion-controlled, the observed ΔE value will be significantly smaller than that predicted by Equation (4).
Quasi-Reversible Processes:
The current peaks become more widely separated
Peak shapes at maxima appear broadened (more rounded)
Peak potentials become scan-rate dependent
ΔE values exceed those given by Equation (4)
Kinetic Analysis Methods:
Regression analysis of the functional relationship between Em (median peak potential) and scan rate (v) can yield both ΔE and k (electron transfer rate constant).
However, using Equation (5) for analysis is considerably more convenient.
(5)
Multi-scan-rate cyclic voltammetry exhibits characteristic curve transformations with increasing scan rates. At lower scan rates, redox reactions proceed more thoroughly at the electrode surface, manifesting as higher current peaks in CV curves. However, this enhanced completeness concurrently intensifies mass transport limitations. Conversely, higher scan rates reduce mass transport resistance but often result in incomplete reactions, yielding diminished current peaks. Fundamentally, this peak current phenomenon stems from competing factors: the controlled variable (applied potential) drives unidirectional change while inherent system forces (e.g., diffusion) create opposing effects. This dynamic competition culminates in peak formation when forward and reverse processes reach transient equilibrium.
The core current equation j = nFAkc reveals that for fixed electrode area (A), peak behavior is governed by two variables:
The reaction rate constant (k), dictated by activation energy (which is potential-dependent)
Reactant concentration (c) at the electrode surface, controlled by mass transport
In fast-kinetic systems, peak generation follows this sequence: During initial potential sweep, reaction consumption outpaces diffusion supply, depleting surface concentration and establishing concentration gradients. As potential progresses, declining surface concentration steepens these gradients until reactant depletion at the electrode surface (c→0) creates maximum concentration gradient—corresponding to peak current (ip). Subsequent potential changes trigger diffusion-layer thickening, gradient relaxation, and current decay as the system transitions to diffusion-controlled operation.
Multi-Scan-Rate CV Testing: Setup Parameters and Obtainable Information
Key Parameters, Configurable Ranges and Typical Settings
The initial potential (scan starting point, typically set at open-circuit voltage) is configurable between -10 V to +10 V. In aqueous systems, this generally ranges within ±2.0 V, while organic systems extend to ±5.0 V; battery systems may require wider ranges. The final potential follows identical configuration principles. For potential limits, the upper vertex potential defines the maximum scan boundary, and the lower vertex potential sets the minimum threshold, both adjustable within ±10 V according to experimental requirements.
Quiet time (stabilization period before scanning) can be set from 1 to 100,000 seconds, though typical experiments use 5-60 seconds. The scan rate (potential change rate) spans 1×10⁻⁴ to 10,000 V/s: steady-state measurements typically employ mV/s ranges (1-50 mV/s), standard electrode studies use 0.01-5 V/s, while ultrafast kinetics research on microelectrodes may reach kV/s regimes. Note that high scan rates induce large currents requiring solution resistance compensation. Cycle number is adjustable from 1 to 500,000 repetitions, with most experiments utilizing 3-50 cycles. The total data points default to 2000 per cycle, resulting in cumulative points = 2000 × cycle number.
Obtainable Information
Cyclic voltammetry at multiple scan rates provides comprehensive electrochemical insights:
(1) Reversibility assessment is determined by symmetry between reduction (Epc) and oxidation (Epa) peaks. Reactions are considered reversible when the peak potential separation satisfies ΔEp≤ 59/n mV (where n = electron transfer number at 298 K).
(2) The formal redox potential (E1/2) is calculated as the average of oxidation and reduction peak potentials.
(3) Electron transfer number (n) can be estimated from both ΔEp and the peak current ratio ( ip,a/ip,c).
(4) Electrochemical rate constants (k⁰) for reversible/quasi-reversible systems are derived through CV curve shape analysis and scan-rate dependent peak current behavior.
(5) Diffusion coefficients (D) are quantified using the Randles-Sevcik equation:
(6) where
ip: peak current (A),
A: electrode area (cm²),
C: bulk concentration (mol/cm³),
v: scan rate (V/s).
Surface active sites involved in adsorption/desorption processes are characterized through CV peak features.
(7) Double-layer capacitance is evaluable within non-Faradaic potential regions, revealing electrode interfacial properties.
(8) Reaction mechanisms are deduced from CV curve morphology, ip-v1/2 relationships, and electrolyte concentration dependencies.
Application Scenarios
Multi-scan-rate cyclic voltammetry (CV) provides critical insights into electrochemical redox processes within batteries, making it indispensable for studying energy storage mechanisms and electrochemical performance. Its key applications in battery technology include:
(1) Electrode Reversibility AssessmentThe bidirectional voltage sweep (cathodic/anodic) enables evaluation of reaction reversibility through oxidation/reduction peak potentials (EpaEpa/EpcEpc) and peak current symmetry in CV curves.
(2) Quantitative AnalysisLinear relationships between oxidation peak currents and analyte concentrations permit quantitative determination of trace intermediates (detection limit: μM-nM range).
(3) Electrode Development• Electrocatalyst Synthesis: CV-enabled fabrication of Pt-Sn/GCE electrodes for ethanol oxidation studies• Advanced Coatings: Electrodeposition of PbO₂ films on graphite substrates for supercapacitor applications
(4) Performance BenchmarkingEvaluation of key metrics:
Cycle stability
Voltage plateau characteristics
Capacitive vs. diffusion-controlled contributions
(5) Reaction Mechanism InvestigationAnalysis of:
Peak multiplicity and broadening
Peak shift dynamics
Ion diffusion kinetics (e.g., Li⁺ intercalation/deintercalation)
(6) Failure DiagnosticsComparative CV analysis across:
Cycle numbers → Capacity fade mechanisms
Scan rates → Structural degradation patterns
(7) Education & TrainingEssential tool for teaching:
Battery operational principles
Electrochemical analysis techniques
(8) StandardizationSupports development of performance testing protocols for industry standards.
(9) Safety TestingPeak separation (ΔEpΔEp) correlates with polarization severity:
Larger ΔEpΔEp → Higher risk of overcharge/thermal runaway
Critical for abuse tolerance validation
(10) Correlation with LSVCV peaks correspond to dQ/dV features but provide superior kinetic information compared to linear sweep voltammetry (LSV).
Conclusion
Multi-scan-rate CV testing stands as an indispensable tool in battery research and development. This technique not only unveils complex internal electrochemical reactions but also empowers researchers to optimize battery materials and architectures, thereby enhancing overall performance. Crucially, it delivers essential insights for:
Performance benchmarking
Reaction mechanism investigation
Failure mode diagnostics
As battery technologies advance, multi-scan-rate CV will remain fundamental to performance optimization and novel battery development.











