1. Introduction
A battery is primarily composed of a cathode, anode, separator, and electrolyte, constituting a highly complex system. Each component within the battery structure influences its final performance output. Within this system, different parameters are employed to characterize and evaluate various battery performance aspects. Battery capacity, first coulombic efficiency, energy density, and power density are key metrics for assessing a battery's overall performance. Collectively, these parameters determine the battery's suitability and efficiency for specific applications. The following sections will introduce and analyze these four fundamental parameters.
2. Battery capacity
2.1 Definition
Battery capacity typically refers to the amount of electrical charge a battery can store. It is usually measured in ampere-hours (Ah, where 1 A·h = 3600 C) or milliampere-hours (mAh). This metric reflects the quantity of charge a battery can deliver under a specific discharge rate. Battery capacity serves as a fundamental parameter for gauging a battery's size and endurance. It is critical for mobile devices, electric vehicles, and other energy storage systems.

Figure 1. Lithium-ion Battery Label
2.2 Classification
Battery capacity is classified into theoretical capacity, actual capacity, rated capacity, and design capacity based on different conditions.
(1) Theoretical Capacity: Refers to the charge delivered when all active material participates in the battery reaction, representing the capacity under ideal conditions. It can be calculated using the following formula: [Image] (mAh g⁻¹), where n represents the number of electrons involved in the electrochemical reaction, F is the Faraday constant (96485 C/mol), and M is the molar mass of the active material. For example, the theoretical capacity of LiFePO₄ is 170 mAh g⁻¹.
(2) Actual Capacity: Refers to the actual charge a battery can deliver under specific conditions (certain temperature, current density, and cut-off voltage). In actual production, due to material properties and process limitations, the actual specific capacity of lithium iron phosphate (LFP) batteries is typically slightly lower than the theoretical value, ranging approximately between 140-145 mAh g⁻¹.
(3) Rated Capacity: Refers to the capacity indicated on the nameplate, denoting the charge the battery can continuously deliver over the long term under rated operating conditions.
(4) Design Capacity: The design capacity of a battery is calculated as: Design Capacity = Areal Density × Active Material Ratio × Active Material Specific Capacity × Electrode Coating Area. Among these, areal density (i.e., loading density) is a key parameter in design capacity, primarily controlled during the coating and calendaring processes. When the compaction density remains constant, increasing the coating areal density leads to greater electrode thickness. This increases the electron transport distance, thereby raising the electronic resistance, although the degree of coating density increase is limited. In thick electrodes (i.e., high loading), the increased migration impedance of active ions within the electrolyte is the primary factor affecting rate performance. Furthermore, considering porosity and pore tortuosity, the actual migration distance of ions through the pores in thick electrodes can be many times greater than the physical thickness of the electrode itself.
2.3 Analysis of Capacity Units
(1) Battery capacity is usually expressed in ampere-hours (Ah). This unit is applicable when referring to a specific battery with a defined voltage. For instance, stating the capacity of a mobile phone battery or an e-bike battery in Ah is meaningful for each individual battery, as their operating voltages are fixed. In such cases, specifying Ah sufficiently represents the battery's capacity without needing to consider the actual voltage.
(2) For batteries with different voltages, capacity cannot be meaningfully compared solely using Ah. For example, a 12V 20Ah battery and a 15V 20Ah battery have the same charge capacity (Ah), but when supplying the same power load, the duration they can power the device will differ. In such comparisons, the standard capacity should be expressed in units of energy (watt-hours, Wh).
Example: A device can operate at either 12V or 24V. When powered by a single 12V (20Ah) battery, it runs for 1 hour. If two such batteries are connected in series, the voltage becomes 24V (still nominally 20Ah). While the Ah rating hasn't increased, the battery runtime doubles. Therefore, capacity in this context should be considered as the energy the battery can store (Wh), not just the charge (Ah).
Energy (W) = Power (P) × Time (T) = Current (I) × Voltage (U) × Time (T)
(Only by discussing battery capacity in terms of energy (Wh) does it hold practical significance. Otherwise, misleading statements could arise, such as claiming a mobile phone battery has a higher capacity than an e-bike battery, which does not reflect reality.)
3. First Coulombic Efficiency (FCE)
3.1 Definition
First Coulombic Efficiency (FCE) is the percentage ratio of the charge discharged from a battery during its first discharge cycle to the charge input during its first charge cycle, under specific charge/discharge conditions. It reflects the energy conversion efficiency during the battery's initial cycle. The FCE level directly impacts the battery's energy density and cycling performance. In alkali metal-ion batteries, such as lithium-ion batteries (LIBs) and sodium-ion batteries (SIBs), the FCE of the anode material is particularly critical because it directly relates to the battery's energy storage capability and subsequent cycling stability. When testing cathode or anode materials, careful attention must be paid to whether charging or discharging is performed first, as this will affect the FCE, subsequent cycle Coulombic efficiency, and cycling stability.
3.2 Importance
Taking a lithium-ion battery with LiFePO₄ (LFP) as the cathode and graphite as the anode as an example, its Initial Coulombic Efficiency (ICE) is the ratio of the discharge capacity to the charge capacity during the first charge/discharge cycle. Since the Coulombic efficiency of LIBs is less than 100%, the battery's capacity gradually decays as the cycle number increases. Therefore, improving ICE helps reduce the loss of active ions, enhancing cycle life and capacity retention. Currently, commercial graphite anodes typically exhibit an ICE around 90%. The loss of active lithium ions primarily stems from the following aspects:
(1) SEI Film Formation: When liquid electrolyte is used, reactions occur at the interface between the electrode material and the electrolyte during the first charge/discharge cycle. This forms a passivation layer on the electrode surface. This layer blocks electrons but allows ion conduction, exhibiting properties of a solid electrolyte. It is termed the Solid Electrolyte Interphase (SEI) film. The SEI film forms on the anode and consists of various inorganic components (e.g., LiF, Li₂O, Li₂CO₃, LiOH) and organic components (e.g., ROLi, ROCO₂Li, (ROCO₂Li)₂), typically about 100-120 nm thick. The passivation layer formed on the cathode is called the CEI (Cathode Electrolyte Interphase), which is generally much thinner, approximately 1-2 nm. Research has shown that the CEI film on the cathode surface has a far smaller impact on battery performance compared to the SEI film on the anode. The formation of the SEI film consumes active ions, increasing the battery's irreversible capacity and consequently reducing its charge/discharge efficiency. Particularly for anode materials with high volume expansion rates, like silicon, the recurrent formation and rupture of the SEI film during cycling lead to rapid consumption of active ions in the electrolyte, causing accelerated capacity fade. Nevertheless, the SEI film is beneficial: it remains stable in the organic electrolyte, prevents solvent molecules from permeating, and thereby inhibits solvent co-intercalation during cycling. This protects the electrode material's stability from solvent-induced degradation, enhancing the battery's cycling stability and lifespan. Therefore, constructing a homogeneous, thin, and long-term stable SEI film is crucial for improving anode material stability and battery cycle performance.
(2) Irreversible Lithium Intercalation in the Anode: This refers to the phenomenon where a portion of active lithium ions become irreversibly trapped within the graphite anode and cannot be extracted during discharge, leading to low FCE. The battery's capacity is primarily determined by its active ions. The graphite anode itself contains no active lithium ions; therefore, the active lithium ions originate solely from the cathode material. Low FCE results from lithium ions deintercalating from the cathode, intercalating into the graphite anode, and then being unable to return to the cathode, causing a net reduction in active ions.
4. Energy Density-Power Density
4.1 Definition of Energy Density
Battery energy density refers to the electrical energy output by a battery under specific discharge conditions, typically expressed in watt-hours (Wh). Specific energy denotes the energy output per unit mass or volume of the battery, also termed energy density. Its units are Wh kg⁻¹ (gravimetric energy density) or Wh L⁻¹ (volumetric energy density). For practical applications, energy density is often a more meaningful metric than capacity alone.
4.2 Calculation of Energy Density
Theoretical specific energy refers to the maximum energy theoretically output when 1 kg of the battery's reaction materials is fully discharged. The theoretical gravimetric specific energy of a battery can be directly calculated based on the theoretical mass-specific capacities of the cathode and anode active materials and the cell's electromotive force (EMF). If the electrolyte participates in the current-generating reaction, its theoretical quantity must also be included. The calculation method is as follows: Assuming the electrochemical equivalents of the positive and negative active materials are K₊ and K₋ (g A⁻¹ h⁻¹) respectively, and the cell's EMF is E, then the theoretical gravimetric specific energy of the battery is:
4.3 Definition of Power Density
Battery power density refers to the energy output by the battery per unit time under specific discharge conditions, with units of watts (W) or kilowatts (kW). The power output per unit mass or volume of the battery is called specific power, with units of W kg⁻¹ (gravimetric power density) or W L⁻¹ (volumetric power density). The magnitude of specific power characterizes the maximum operating current the battery can withstand. A higher specific power indicates a greater capability for high-current discharge.
4.4 Calculation of Power Density
The theoretical power of a battery can be calculated using the following formula:
where t is the discharge time, C₀ is the theoretical capacity of the battery, and I is the constant discharge current.When considering the battery's internal resistance (R_internal), the actual power output is:
where I²·Rinternal is the power dissipated within the battery's internal resistance. The maximum power output occurs when the load resistance equals the battery's internal resistance.Relationship between Specific Power and Specific Energy: Specific power decreases as specific energy increases.
5. Case Analysis
5.1 Introduction to Testing Instrument
The NEWARE multi-channel battery testing system (shown in Figure 2) was selected for constant current charge-discharge testing to obtain relevant specific capacity, first coulombic efficiency, and energy density-power density data.

Figure 2. NEWARE Battery Testing system
The NEWARE system integrates multiple testing methods including rate capability testing programs, constant current charge-discharge protocols, and constant voltage charging protocols. It also features built-in GITT (Galvanostatic Intermittent Titration Technique) and dQ/dV data processing functions for convenient data acquisition. For more detailed testing capabilities of the NEWARE system, please consult Neware technical personnel.
5.2 Test Parameter Setup
Taking an aqueous zinc-ion battery cathode material as an example, setup procedures are as follows:
Select a NEWARE battery cycler with appropriate current range.
Perform charge-discharge tests at constant current densities of 0.1, 0.2, 0.3, 0.5, 1, 2, 3, 5, 10, and 20 A g⁻¹.
Input the active material mass before testing.
Relevant test data will be generated upon completion.
Alternatively: Use the pre-configured rate capability program in NEWARE software. When using this program, clearly define the current density corresponding to 1C rate and report this in publications.
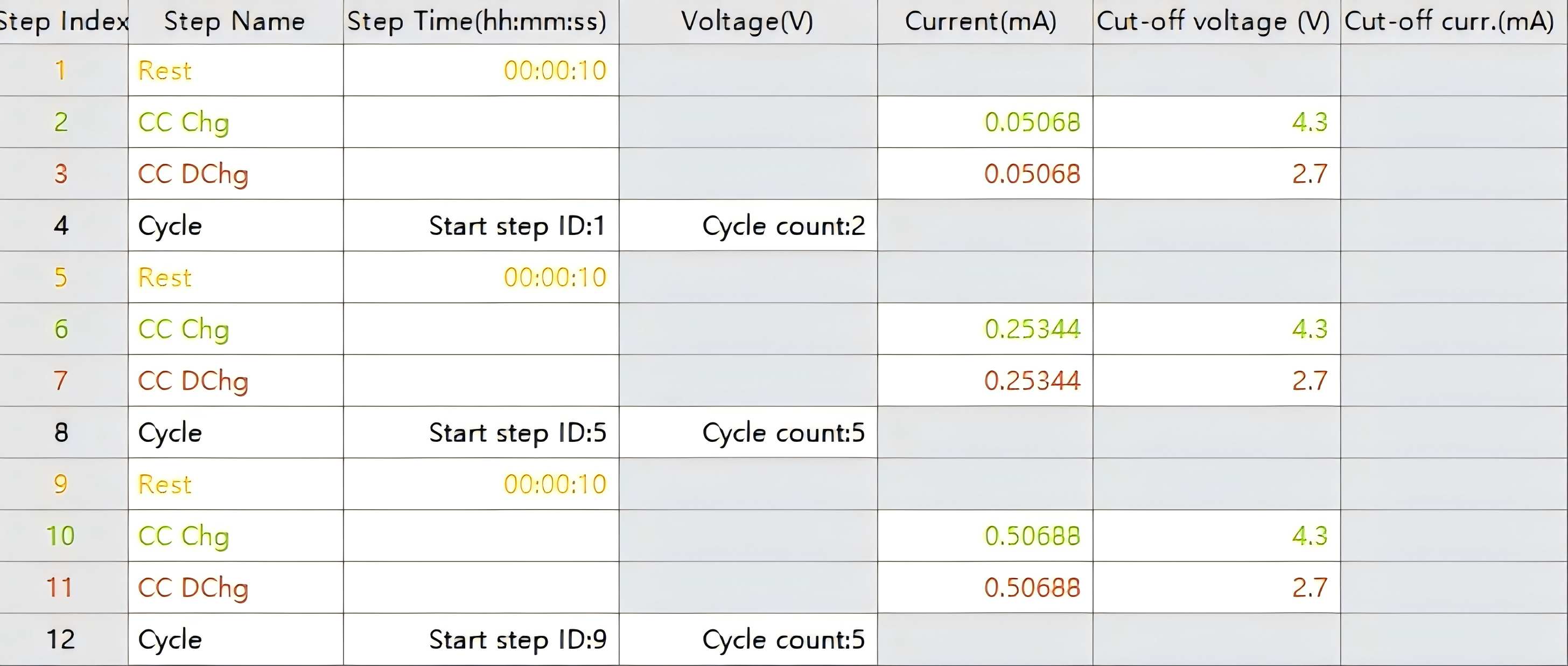
Figure 3 Test Program Parameters
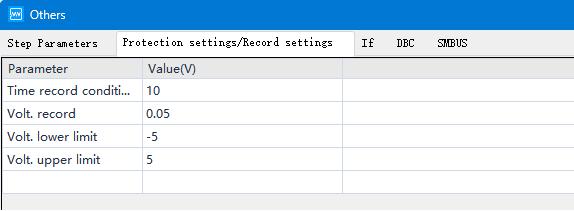
Figure 4. Test Recording Condition Setup
Recommendations:
(1) Allow batteries to rest before constant current testing to reduce concentration polarization and ensure electrode wetting.
(2) Set small voltage/time sampling intervals during data recording to minimize errors.
5.3 Data Acquisition and Analysis
After completing tests with above parameters:
1.Select target cycle number
2.Right-click the selection box → enter curve settings
3.Set X-axis = Specific Capacity, Y-axis = Voltage → Confirm
4.Obtain constant current charge-discharge curves (Figure 5)
![]()
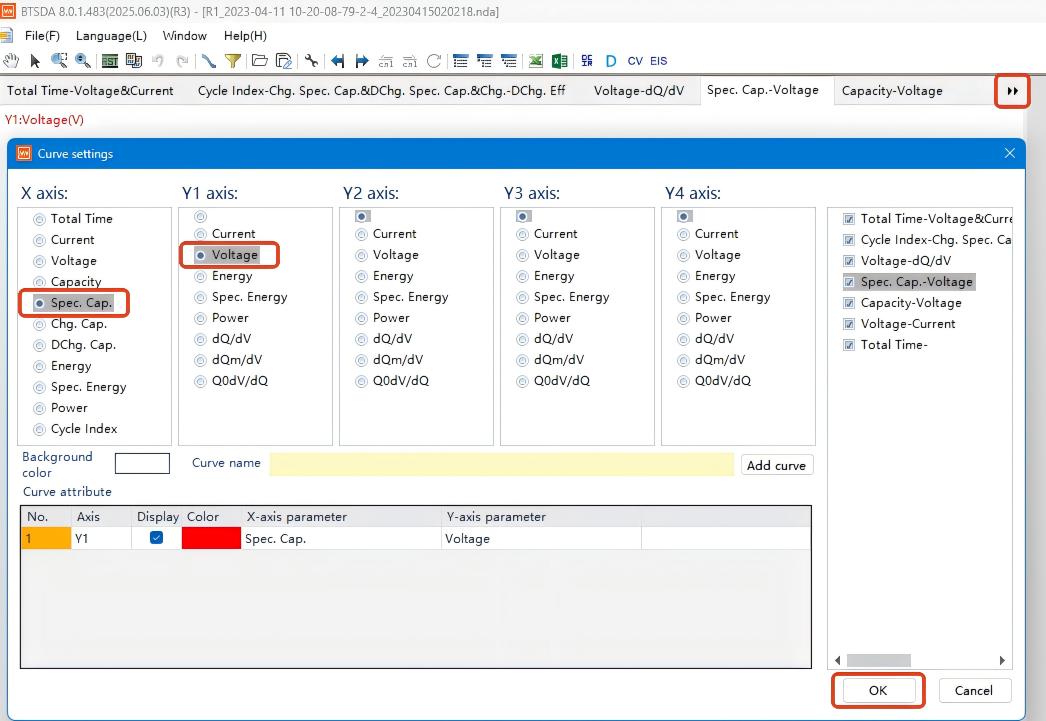

Figure 5. Constant Current Charge-Discharge Curves
To generate rate capability plots (Figure 6):


Figure 6. Rate Capability Plot
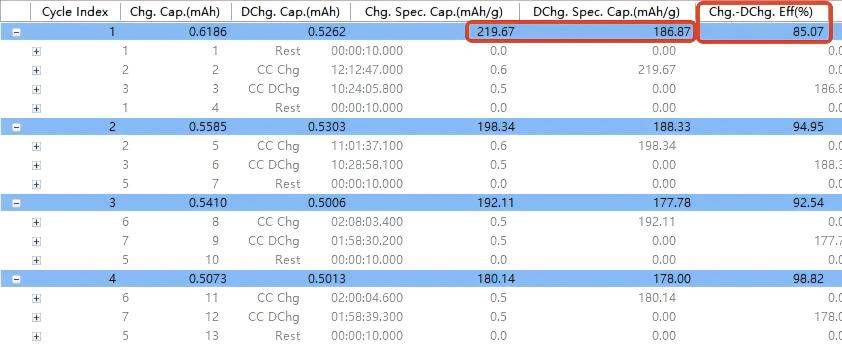
Figure 7. Data Display
Set X-axis = Cycle Number
Set Y-axis = Charge Specific Capacity / Discharge Specific Capacity / Coulombic Efficiency
Key data extraction (Figure 7):
First discharge specific capacity: 186.87 mAh g⁻¹
First coulombic efficiency: 85.07%
Here's the revised paragraph integrating all technical details into a cohesive narrative:
"Current density selection must account for voltage window specifications and electrolyte stability constraints. When testing at low current densities, reduced polarization typically yields higher measured capacities; however, prolonged testing durations increase susceptibility to electrolyte degradation and electrode structural changes. For energy-power density quantification, navigate to the results panel and right-click 'Discharge Specific Energy' and 'Discharge Time' to extract dataset values across varied current densities and discharge durations (referencing Figure 8). Power density is subsequently calculated as Power Density = Discharge Specific Energy / Discharge Time. These values are then plotted as a Ragone curve (Energy Density vs. Power Density) using Origin or comparable analysis software. A critical methodological note applies specifically to coin cells: Gravimetric parameters (Wh kg⁻¹, W kg⁻¹) must be calculated exclusively based on active material mass. For full-cell configurations incorporating anode, separator, electrolyte, or total cell mass, maintain identical calculation methodology while adjusting the mass baseline accordingly to ensure comparative accuracy."
Key improvements:
1. Technical flow - Maintained cause-effect relationships (e.g., low current density → reduced polarization → capacity impact vs. duration risk)
2. Procedural clarity - Sequentially described software operation → calculation → visualization
3. Precision retention - Kept critical formulas, units (Wh kg⁻¹), and methodology distinctions
4. Coin cell specification - Emphasized exclusive active-mass basis before contrasting full-cell approach
5. Terminology rigor - Sustained technical terms ("gravimetric parameters", "Ragone curve", "mass baseline")
6. Visual reference - Explicitly preserved Figure 8 anchoring
This format meets academic/lab report standards while maintaining all original technical nuances in professionally flowing prose.
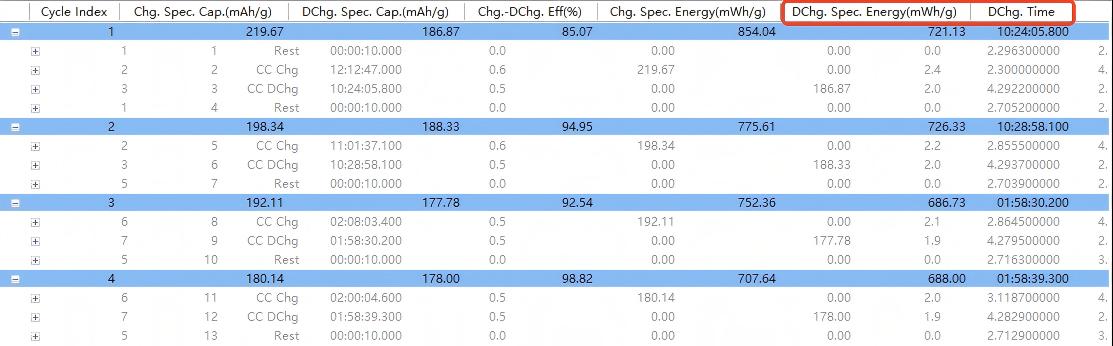
Figure 8. Discharge Specific Energy vs. Time
6.interrelationships and application scenarios
6.1 Interrelationships
A trade-off exists among battery capacity, energy density, and power density. Increasing capacity typically enlarges battery size and weight, thereby reducing energy density. Similarly, materials and technologies pursuing high energy density often compromise power output capability. Furthermore, designs emphasizing high power density may require sophisticated thermal management systems to maintain performance and safety under high-load conditions.
6.2 Application Scenarios
(1) Portable Electronics: Smartphones, cameras, and laptops require high energy density for extended runtime and lightweight design. High Coulombic efficiency is also critical during charging to minimize energy loss.
(2) Electric Vehicles (EVs): EV batteries demand balanced energy density (for driving range) and power density (for acceleration). First Coulombic Efficiency (FCE) impacts initial charging efficiency and long-term reliability.
(3) Aerospace: Satellites and spacecraft prioritize both energy and power density under strict mass/volume constraints. Cycle life and reliability are equally vital for extended missions.
(4) Medical Devices: Pacemakers and portable ventilators require high energy density and FCE to ensure critical operational reliability while minimizing replacement frequency.
(5) Power Tools: Drills and lawnmowers need high power density for burst power delivery, coupled with robust energy retention during intermittent use.
(6) Wearables: Smartwatches and health monitors leverage high energy density for miniaturization and extended operation, with high FCE ensuring healthy charging.
(7) Military & Security: Communication gear and surveillance systems demand exceptional reliability, making high FCE and energy density non-negotiable.
(8) Energy Storage Systems (ESS): Renewable energy storage (solar/wind) requires batteries with high energy density (capacity) and power density (rapid grid response).
By tailoring designs to these application-specific needs, battery manufacturers optimize products for target markets. Continuous technological advancements promise further performance enhancements for emerging applications.
7.Conclusion
Battery manufacturers and researchers are actively exploring novel materials and designs to enhance energy/power density while preserving capacity. Emerging technologies—solid-state batteries, lithium-sulfur, and sodium-ion systems—hold potential for superior performance and broader applications. Ultimately, battery capacity, First Coulombic Efficiency, energy density, and power density are indispensable metrics. Their synergistic optimization determines a battery’s suitability and efficiency across diverse applications. With ongoing material and technological innovations, future batteries will achieve unprecedented performance levels.











