1. Introduction
The diffusion of active ions within electrode materials constitutes a critical reaction process. This bulk diffusion is typically slow and often becomes the rate-limiting step, affecting the overall reaction kinetics and determining the battery's performance. A higher ionic diffusion coefficient indicates faster ion transport within the material, leading to superior high-current charge/discharge capability, improved power density, and enhanced rate performance of the electrode. Therefore, studying the variation of the diffusion coefficient during diffusion processes is essential for modifying and optimizing the electrochemical properties of materials.
In methodologies for investigating ionic diffusion coefficients, Fick's first and second laws primarily serve as the foundational framework for calculations. By applying small-amplitude direct current (DC), alternating current (AC), or voltage signals to the electrode, the corresponding electrochemical response signals are obtained, enabling linearization treatment of the diffusion process. Current primary techniques for measuring ionic diffusion coefficients include:
Galvanostatic Intermittent Titration Technique (GITT)
Potentiostatic Intermittent Titration Technique (PITT)
Cyclic Voltammetry (CV)
Electrochemical Impedance Spectroscopy (EIS)
Potential Relaxation Technique (PRT)
GITT testing offers distinct advantages by mitigating interference from resistive polarization and demonstrating greater operational simplicity. Consequently, this article focuses on elucidating the fundamental principles and step configuration of GITT for determining ionic diffusion coefficients in batteries.
2. Principles and Methodology of GITT Testing
2.1 Principles
GITT (Galvanostatic Intermittent Titration Technique) is a transient electrochemical technique. Its fundamental principle involves applying a constant current ii to the battery for charging or discharging over a fixed duration ττ, followed by terminating the current. The voltage response is recorded throughout both the galvanostatic phase and the subsequent relaxation period. This recorded voltage data serves as the basis for analyzing the polarization behavior associated with the electrode reaction, thereby enabling the calculation of reaction kinetics.
The GITT test comprises a sequence of alternating "current pulse → galvanostatic phase → relaxation period" cycles. The relaxation period refers to the interval during which no current passes through the battery.
The current intensity ii and the relaxation time ττ constitute critical parameters in GITT experimental design.
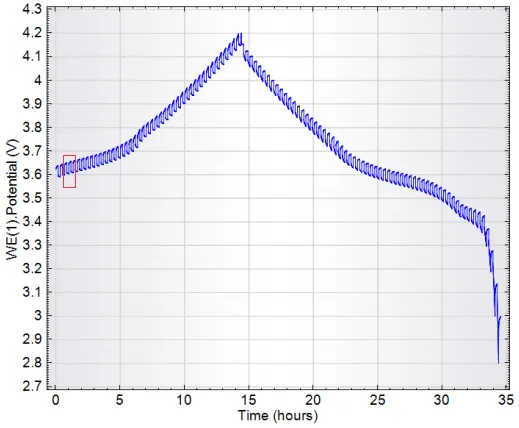
Figure 1. GITT Profile of a Commercial Lithium-Ion Battery
Figure 1 shows the GITT profile of a commercial lithium-ion battery, with Figure 2 presenting an enlarged view of its selected region.
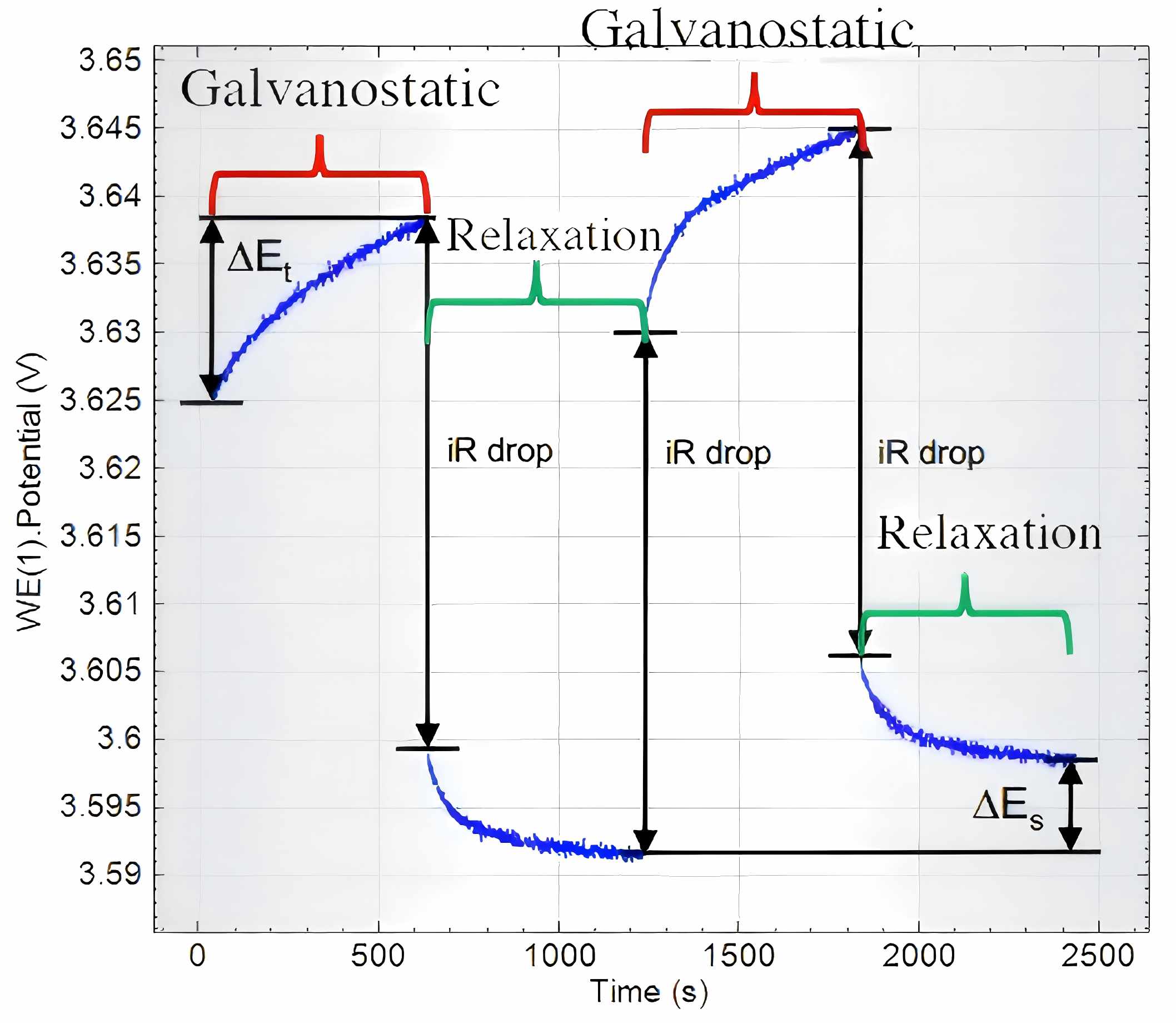
Figure 2. Enlarged View of GITT Profile Region
2.2 Mathematical Formulations for GITT Analysis
In the equation, D denotes the diffusion coefficient, i represents the applied current, F is the Faraday constant (96485 C/mol), zA indicates the charge number of the ion, S corresponds to the electrode/electrolyte contact area, and dE/dδ signifies the slope of the coulometric titration curve. The term potential versus time profile describes the functional relationship between potential and time. To simplify the solution, when the applied current i is sufficiently small and the relaxation time τ is sufficiently short, the profile exhibits a linear relationship. Consequently, the preceding equation can be simplified to the following expression:
In the equation:
τ denotes the duration of the applied constant current pulse (s)
mB represents the mass of active material (g)
Vm indicates the molar volume of the sample (cm³/mol)
MB corresponds to the molecular weight of the material (g/mol)
S signifies the electrode/electrolyte interfacial contact area (cm²)
ΔEs refers to the steady-state voltage change during the charging/discharging process (V)
ΔEt designates the voltage variation upon relaxation to equilibrium state (V)
L defines the electrode thickness (cm)
3. Experimental Setup for GITT Testing
3.1 Overview of Testing Instrumentation
Based on the fundamental principles and computational methodology of GITT, the ionic diffusion coefficient DD can be determined through parameter-optimized testing followed by data analysis. The NEWARE Multi-channel Battery Testing System (illustrated in Figure 3) was employed for GITT measurements in this study.

Figure 3. NEWARE Battery Testing System
The NEWARE Multi-channel Battery Testing System integrates numerous operational modes:
1. Charging Modes
Constant Current (CC) Charging
Constant Voltage (CV) Charging
CC-CV Charging
Constant Power (CP) ChargingTermination Conditions: Voltage, Current, Relative Time, Capacity, Energy, -ΔV
2. Discharging Modes
Constant Current (CC) Discharging
Constant Voltage (CV) Discharging
CC-CV Discharging
Constant Power (CP) Discharging
Constant Resistance (CR) DischargingTermination Conditions: Voltage, Current, Relative Time, Capacity, Energy
3. Pulse Testing
Charge Pulses: CC / CP modes
Discharge Pulses: CC / CP modes
Minimum Pulse Width: 500 ms
Pulse Sequences: Supports 32 distinct pulses per step
Seamless Charge-Discharge Transition: Direct switching within a single pulse stepTermination Conditions: Voltage, Relative Time
4. DC Internal Resistance (DCIR) Testing
Supports user-defined sampling points for DCIR calculation
5. Cycling Parameters
Cycle Range: 1–65,535 cycles
Steps per Cycle: Up to 254
6. Nested Cycling
Multi-layer cycle nesting (max. 3 levels)
(For detailed specifications, contact NEWARE application engineers.)
3.2 Test Parameter Configuration
Using aqueous zinc-ion battery vanadium-based cathode materials as an exemplar system, the GITT procedure follows these operational steps:
Apply a constant current discharge for 5 minutes
Implement a 2-hour rest period until equilibrium is attained
Iteratively repeat this sequence until reaching the predetermined cutoff voltageNote: The charging protocol mirrors the discharge procedure.
Recommendations:
Conduct 2-5 pre-cycling cycles prior to GITT measurements
Employ the lowest current rate used in rate capability tests (typically 0.05-0.2C) or lower
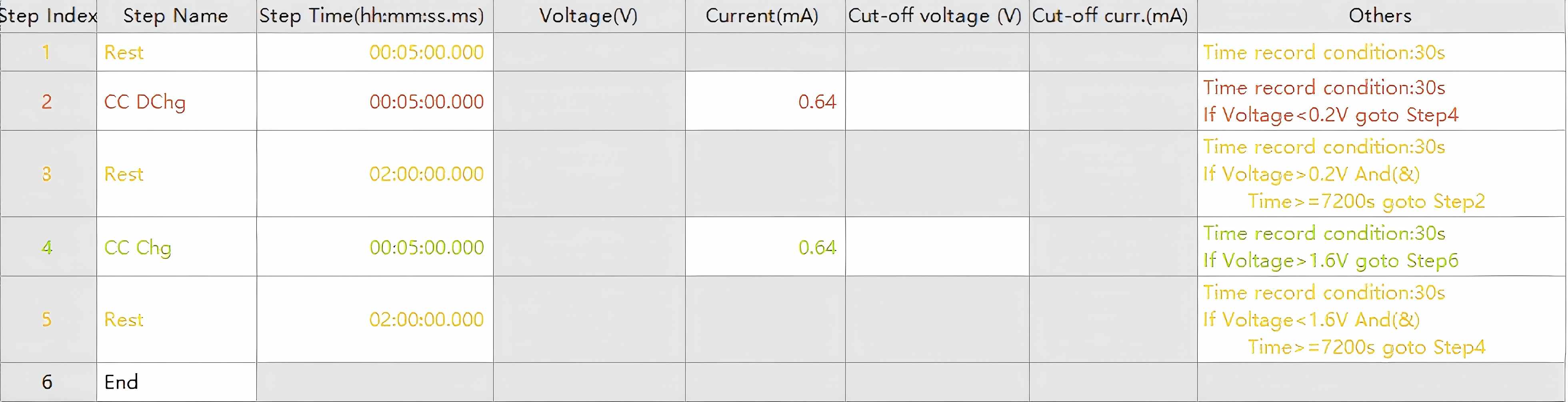
Figure 4. GITT Testing Protocol Schematic
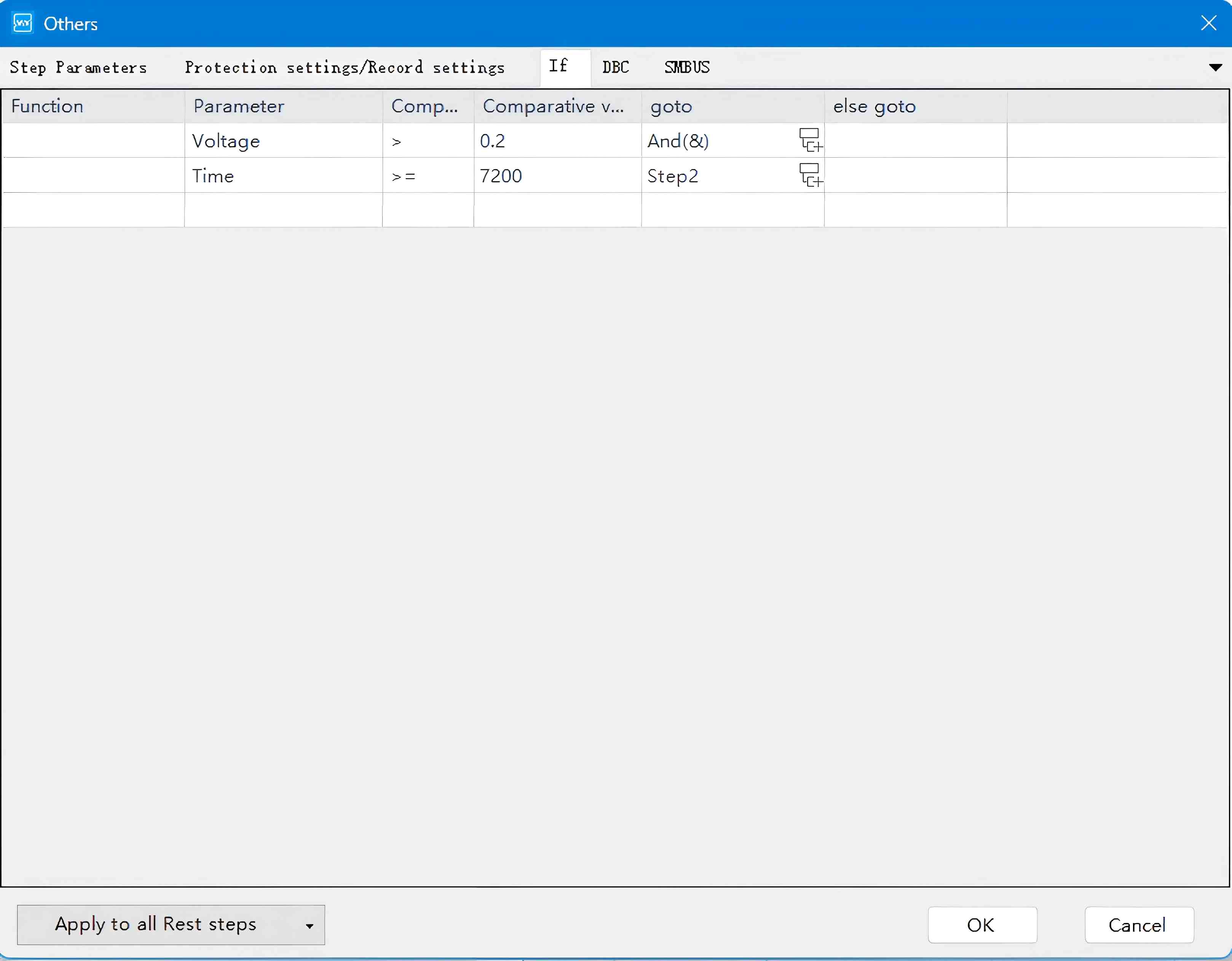
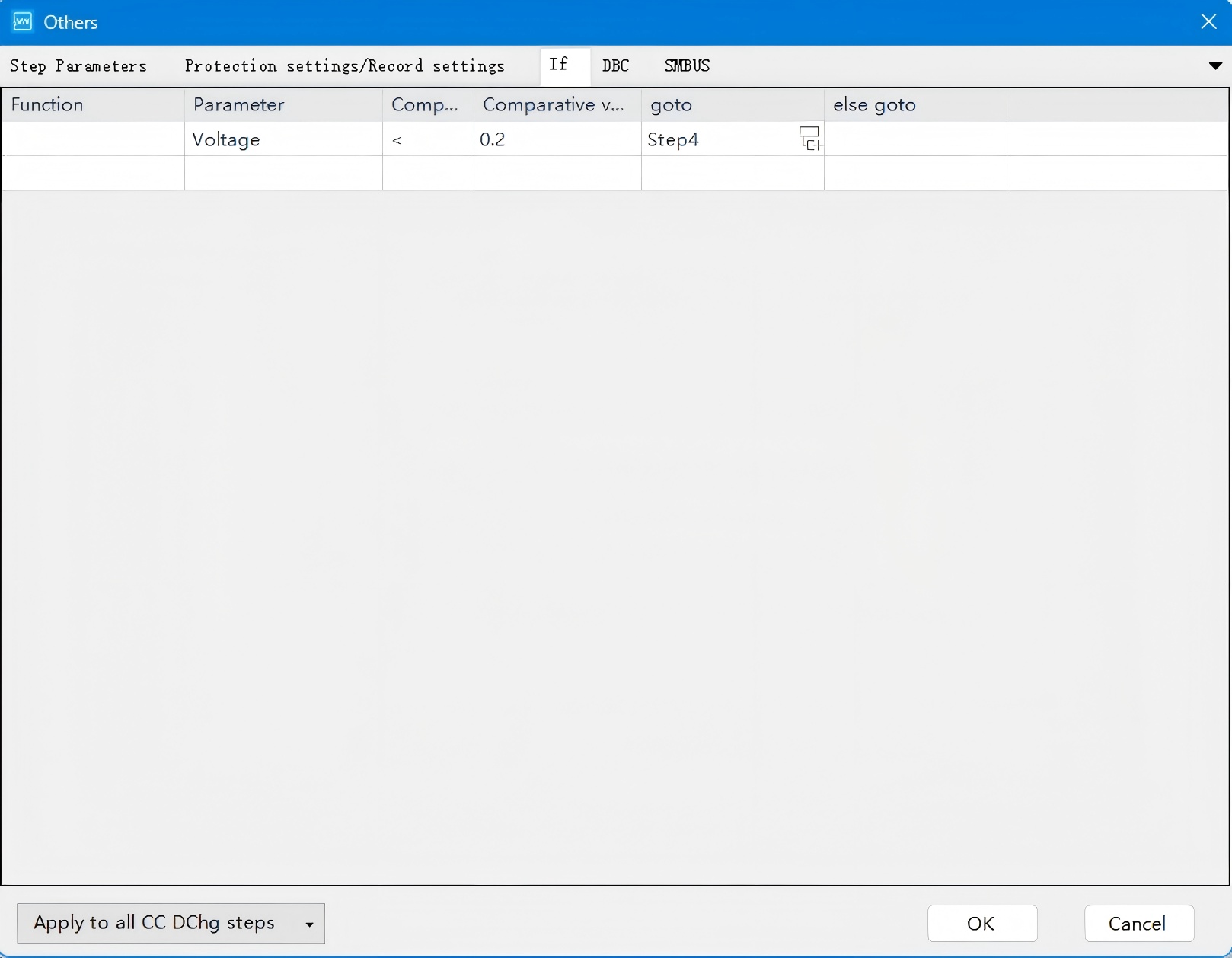
Figure 5. Configuration of GITT Data Acquisition Parameters
Upon completion of the test configured with the aforementioned parameters, the characteristic GITT profile as shown in Figure 6 is obtained.
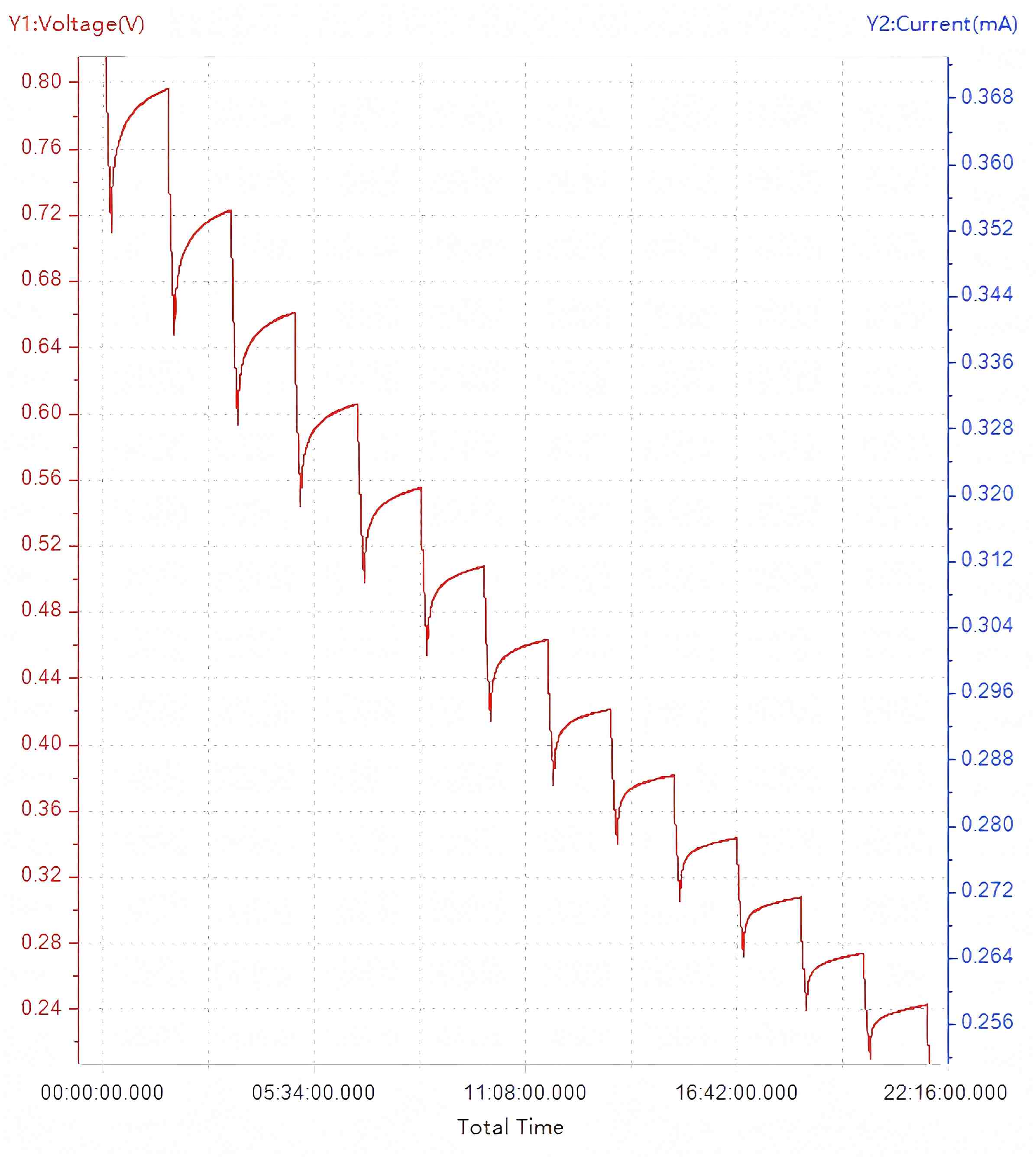
Figure 6. GITT Voltage Profile
An enlarged view of the discharge phase from Figure 6 is presented in the representative plot of Figure 7.
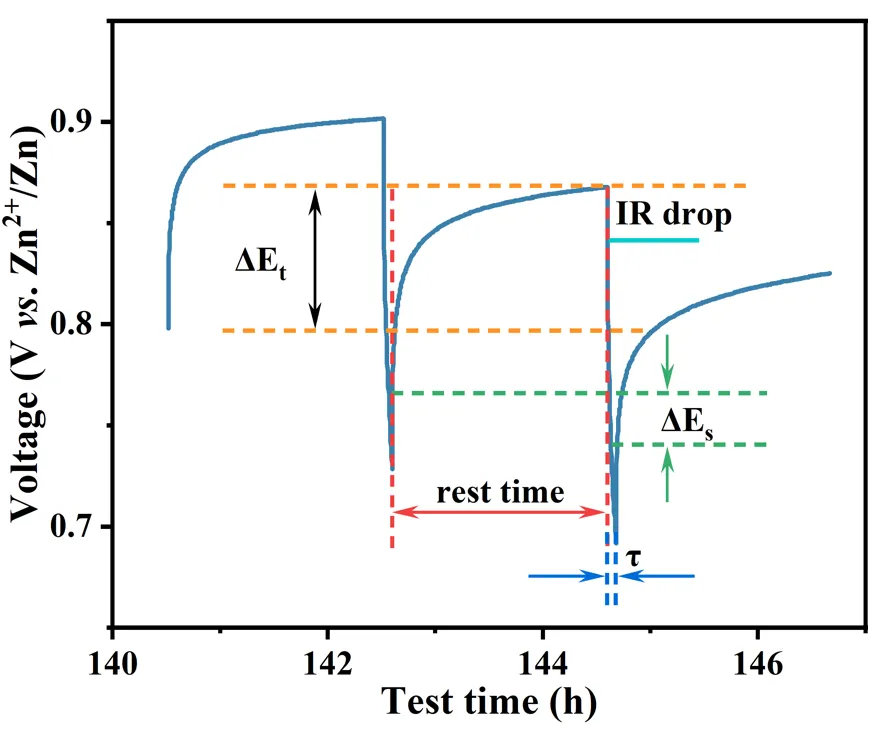
Figure 7. Partial enlarged view of GITT
3.3 Test Data Analysis
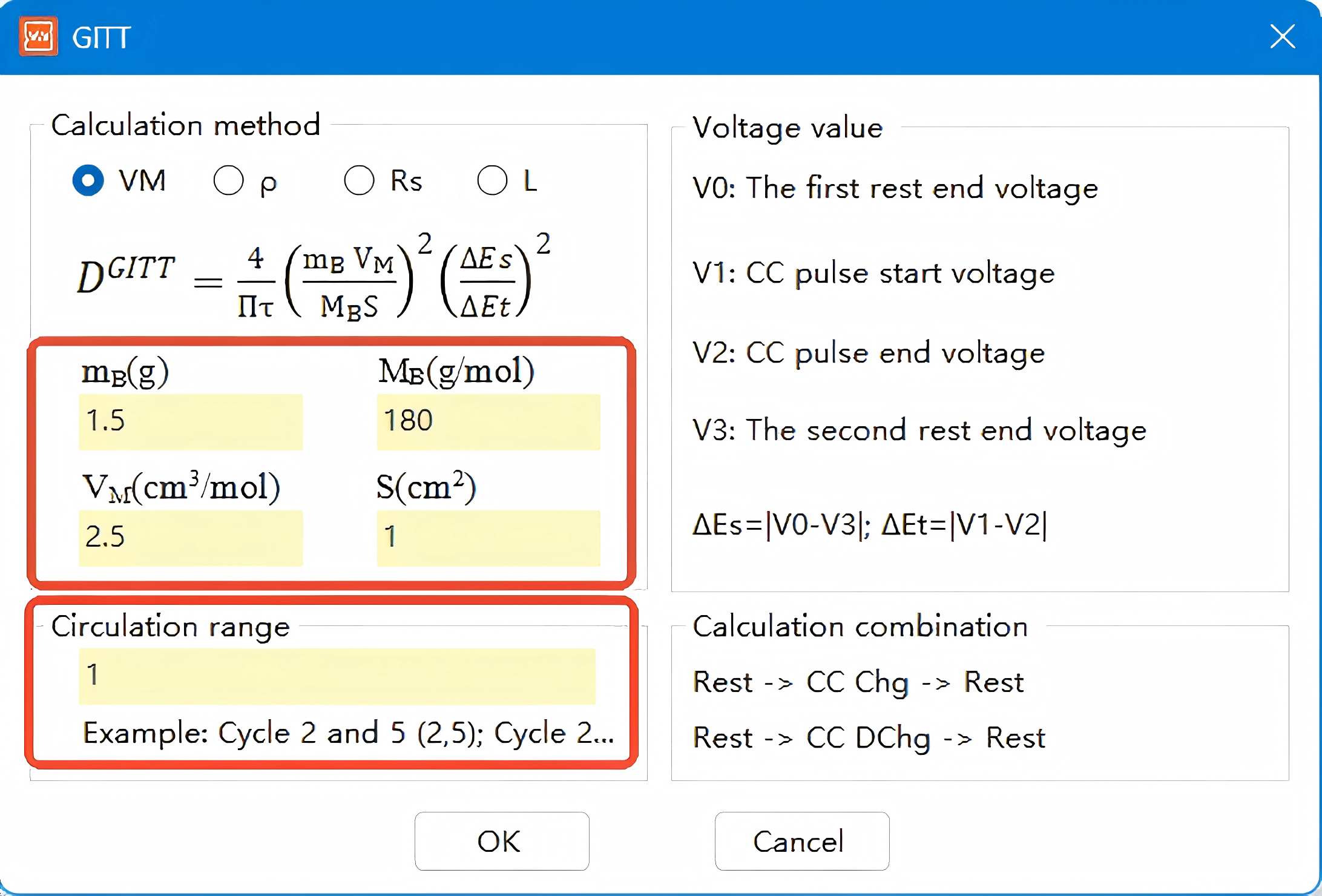
Access the GITT dataset acquired through the NEWARE multi-channel battery testing system and select the letter 'D' in the interface to initiate the GITT data processing module.
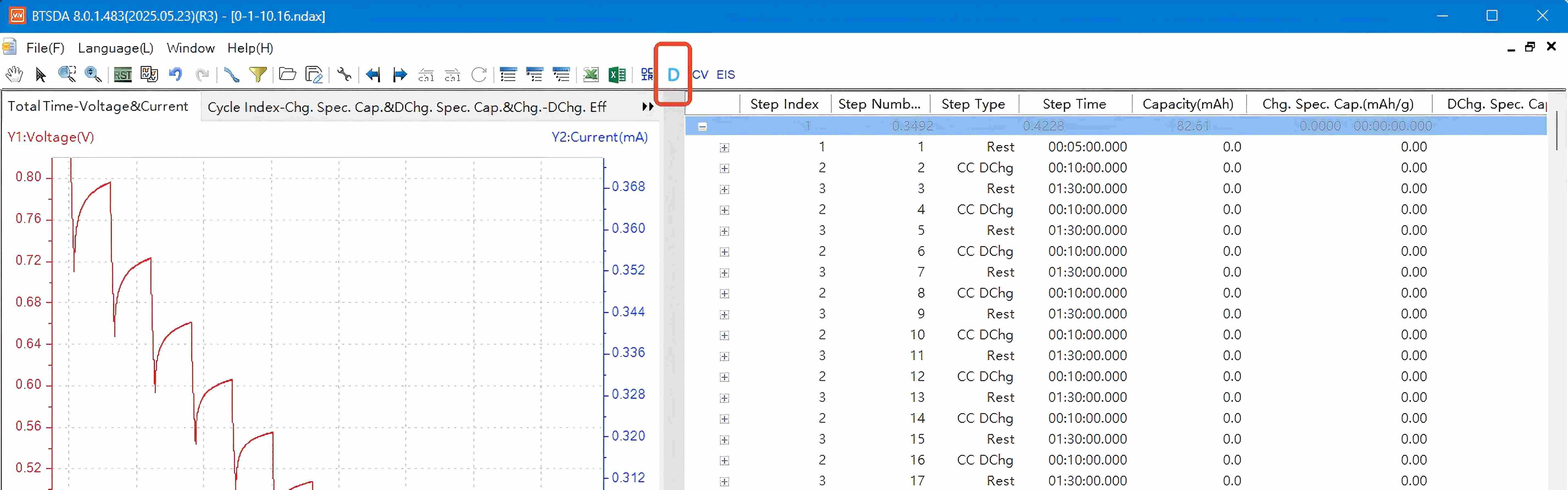
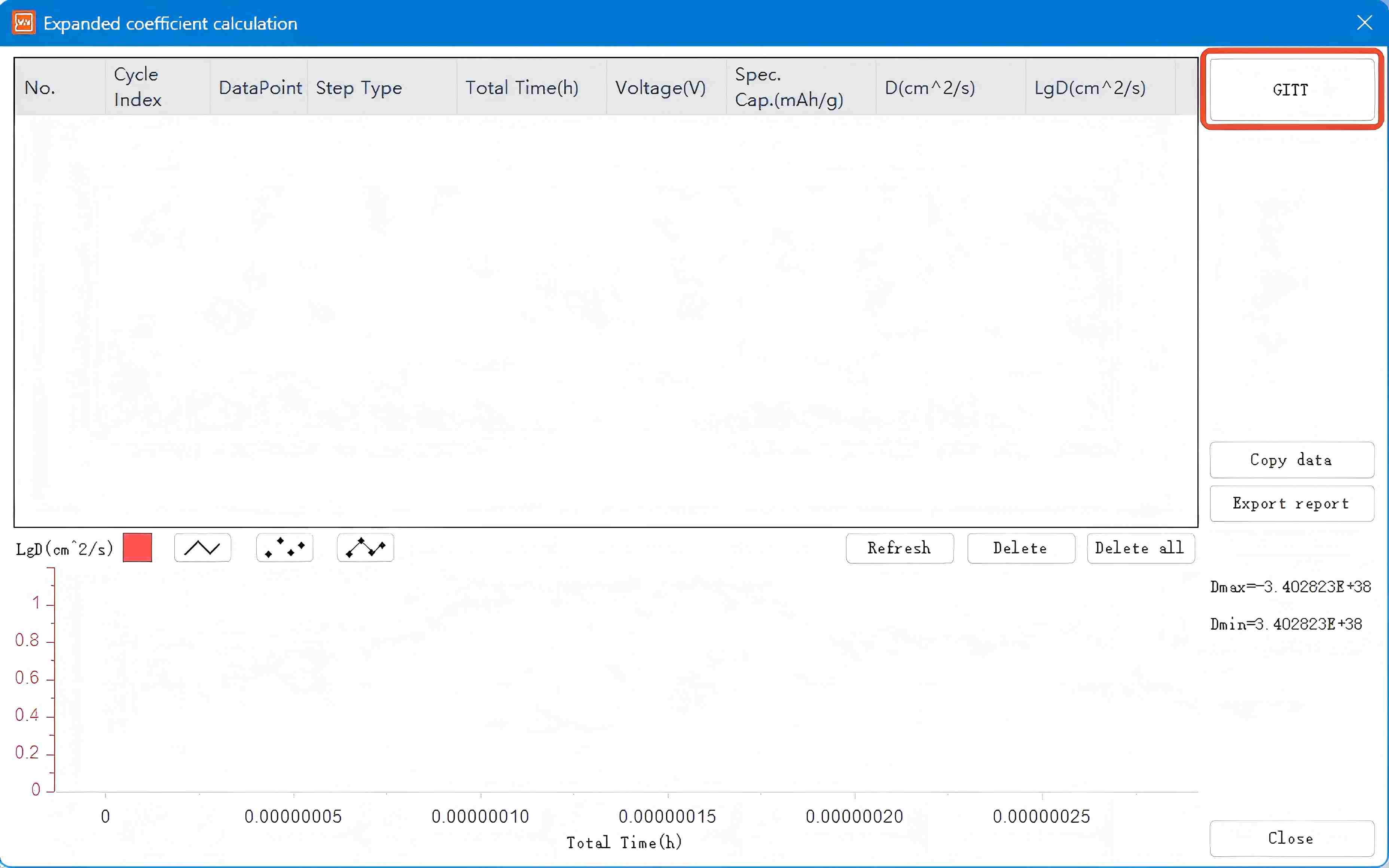
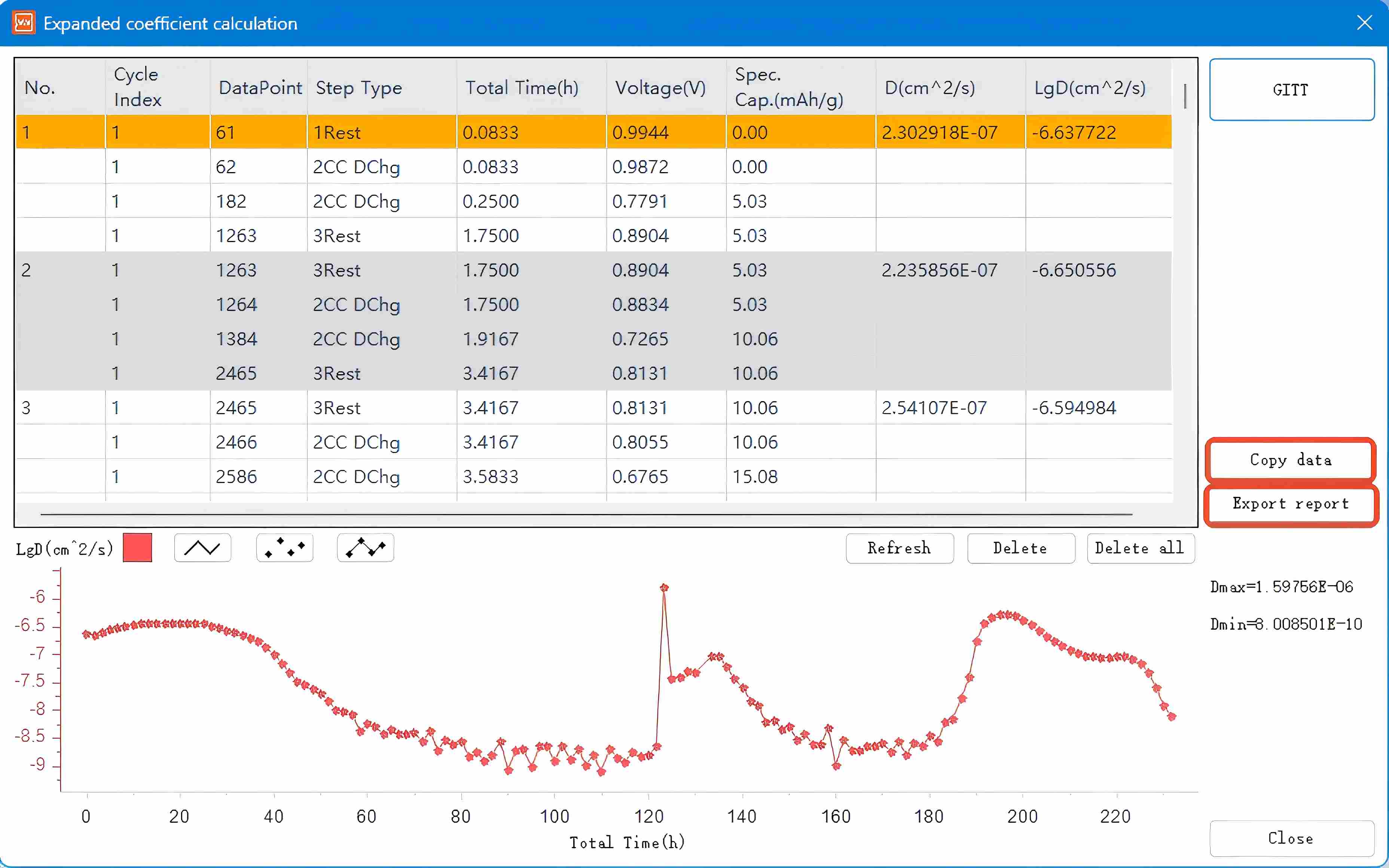
Click the GITT button here to enter the Material Parameter Setting interface. Input the parameters in the indicated area based on calculations for the studied material, then click Determine to generate the GITT processed data graph. Copy the data to Origin software to plot the corresponding diffusion coefficient graph.

4. Application Scenarios
Ion diffusion coefficient is a critically important factor in studying electrochemical performance and enhancing the overall performance of batteries. Consequently, the Galvanostatic Intermittent Titration Technique (GITT) finds widespread application in electrochemistry, particularly in battery research. Specific application scenarios include the following areas:
Electrode Material Design: By measuring ion diffusion coefficients, researchers can understand the ion transport properties of different electrode materials. This enables the design and optimization of materials with faster ion transport rates, improving the battery's power density and charge/discharge rate capability.
Battery Performance Evaluation: The ion diffusion coefficient helps evaluate battery performance under various operating conditions, such as high-rate charge/discharge capability. A high diffusion coefficient indicates better performance under high-current operation.
Battery Cycle Life Prediction: Measuring the ion diffusion coefficient can reveal the stability of materials during charge/discharge cycles. This is crucial for predicting battery cycle life and understanding aging mechanisms.
Battery Thermal Management: The ion diffusion process can be accompanied by heat generation. Understanding the ion diffusion coefficient aids in optimizing battery thermal management design, preventing overheating, and ensuring safety.
Battery Modeling and Simulation: In electrochemical modeling and simulation of batteries, the ion diffusion coefficient is an essential parameter. It is vital for simulating battery charge/discharge behavior and predicting battery response.
Battery State Monitoring: Monitoring changes in the ion diffusion coefficient during charge/discharge cycles allows for real-time assessment of battery health status (SOH) and performance degradation.
New Material Development: When developing novel battery materials, measuring the ion diffusion coefficient serves as a key metric for screening and evaluating material performance, accelerating the transition of new materials from the lab to commercialization.
Battery Management Systems (BMS): BMS requires accurate knowledge of the internal ion diffusion state within the battery to optimize charge/discharge strategies, extend battery life, and improve usage efficiency.
Battery Recycling and Reuse: During the battery recycling process, measuring the ion diffusion coefficient of used batteries helps assess their remaining value and reuse potential.
Battery Safety Research: Understanding the ion diffusion mechanisms within battery materials aids in identifying risk factors that could lead to battery failure and thermal runaway, thereby enabling the development of safer battery systems.
Through the above application scenarios, it is evident that the ion diffusion coefficient is a multifaceted key parameter influencing various aspects of battery performance. It has a profound impact on the advancement of battery technology. Therefore, GITT testing is indispensable for obtaining this critical diffusion coefficient.
5. Conclusion on GITT Methodology
In summary, the GITT diffusion coefficient testing method enables:
Accurate measurement of diffusion coefficients
Simulation of actual operating conditions
Non-destructive testing (NDT) capability
Comprehensive and adaptable implementation across diverse battery systems
While GITT provides critical insights into ion diffusion behavior of battery materials, it presents certain limitations and challenges, including time-consuming procedures and demanding equipment specifications. Researchers must carefully consider these factors to ensure testing validity and result accuracy.
Transition to Future Content
This article has provided foundational insights into GITT testing principles, parameter configuration, and data processing. In upcoming installments of our Electrochemical Testing Series, the Neware Team will detail specialized testing methodologies for battery materials and design, including:
Rate capability performance
Energy efficiency analysis
dQ/dV curve interpretation
Charge/discharge curve diagnostics
Stay tuned for these advanced technical explorations. We welcome your continued engagement, sharing, and support of our scientific endeavors.