Overview of cathode materials for sodium-ion batteries
Sodium-ion batteries share a similar structure, components, and charge-discharge mechanisms with lithium-ion batteries. In recent years, lithium-ion batteries have developed rapidly and become the dominant battery technology in the market. However, significant progress has also been made in the research of sodium-ion batteries, a newer and more cost-effective alternative. Similar to lithium-ion batteries, the cathode material is the most critical component determining the performance of sodium-ion batteries. The physicochemical and electrochemical properties of catho materials, such as particle size, morphology, specific surface area, tapping density, structure, and composition, are significantly influence the application of sodium-ion batteries. Therefore, this article will provide an introduction to the cathode materials used in sodium-ion batteries.
Characteristics of ideal cathode materials for sodium-ion batteries
An ideal cathode material for sodium-ion batteries should possess the following advantages:
High operating voltage: The Gibbs free energy during the battery discharge reaction should be sufficiently large, resulting in a high open-circuit voltage and thereby delivering a higher specific capacity.
High theoretical specific capacity: The material should accommodate more sodium ions per unit mass, enabling reversible intercalation and deintercalation. Transition metal ions should exhibit variable oxidation states to maintain charge balance during charge-discharge cycles.
Long cycle life: The structural changes during sodium ion insertion/extraction should be fully reversible to ensure the material's integrity remains intact.
Excellent rate capability: The sodium-ion diffusion coefficient should be high, with fast diffusion rates both inside and on the surface of the electrode material.
Good chemical stability: The material should undergo minimal chemical reactions with the electrolyte during battery storage and operation.
Simple and environmentally friendly preparation process.
Relatively high electronic and ionic conductivity.
Classification and introduction of cathode materials for sodium-ion batteries
Currently, the mainstream cathode materials for sodium-ion batteries are the following three types:
Layered oxides (NaMxO2, where M represents one or multiple transition metal elements)
Polyanionic compounds (e.g., NaFePO4, Na3V2(PO4)3)
Prussian blue analogues (e.g., NaFe[Fe(CN)6])
Layered oxides
The general chemical formula for layered oxides can be expressed as NaxMO2 (where "x" represents the sodium content with 0
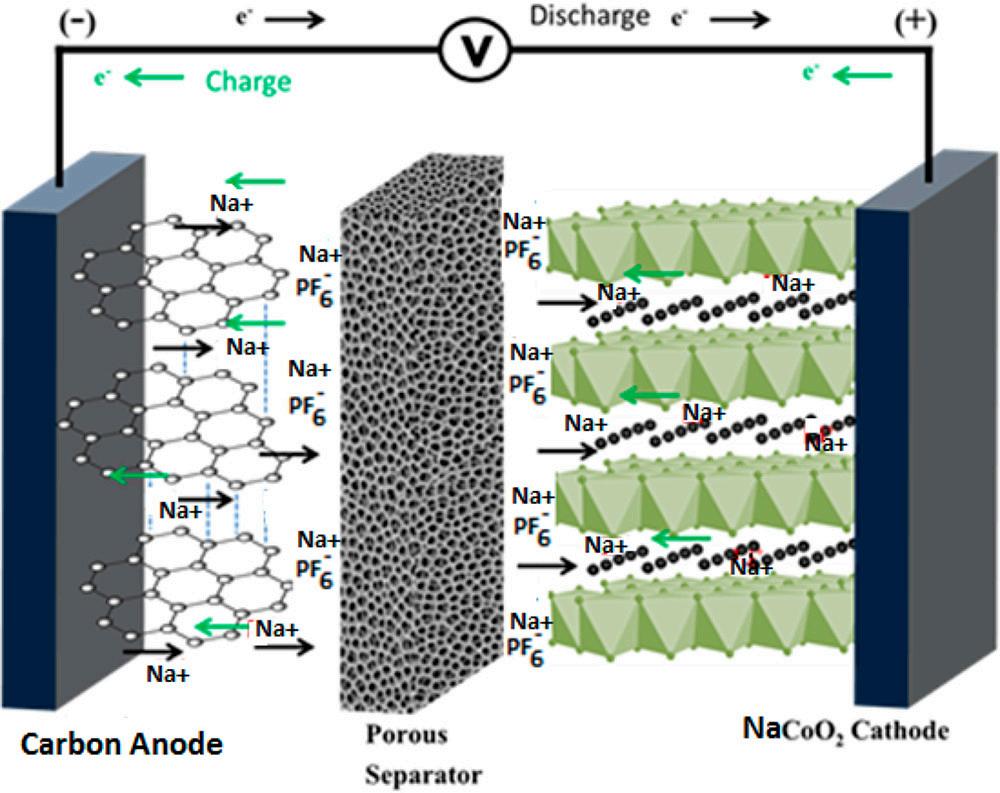
Figure 1 The charging and discharging schematic diagram of NaCoO2[1]
Transition metal elements possess multiple valence states, enabling redox reactions during charge and discharge processes, thereby facilitating the reversible intercalation and deintercalation of sodium ions. Different transition metal elements impart distinct effects on material properties. For instance, cobalt enhances electronic conductivity and structural stability, manganese reduces material costs, and nickel improves energy density. Layered oxide cathode materials exhibit an actual specific capacity of 140–160 mAh/g and an average operating voltage of 3.2V–3.5V. Although their specific capacity is lower than that of layered lithium-ion batteries (e.g., ternary cathodes), sodium-ion batteries offer significant advantages, including a broad operating temperature range (–40℃ to 60℃), the use of aluminum foil for anodes (reducing cell mass and improving pack energy density), and enhanced safety. Furthermore, their manufacturing process is compatible with existing lithium-ion battery cathode production systems. Comprehensive testing equipment and systems capable of wide-temperature-range evaluations (e.g., the NEWARE WGDW series, supporting environments from –70℃ to 150℃, and chamber volumes from 100L up to 1000L. Figure 2) have also been developed. Given these benefits, sodium-ion batteries utilizing layered oxide cathodes hold considerable market potential.

Figure 2 High and low temperature chamber from NEWARE
Similar to the high-nickel lithium battery cathode materials used in current high-end markets, high-nickel layered sodium-ion cathode materials (e.g., NCM811) incorporate a high Ni content to replace expensive Co, reducing costs while leveraging Ni to deliver higher capacity. Mn helps maintain the structural framework stability, and Co contributes to stabilizing the structure while enhancing the average operating voltage of the battery. However, under high voltage during deep desodiation, high-nickel materials are prone to irreversible phase transitions, such as from O3 to P3 (as shown in Figure 3) or more complex structural changes. This leads to alterations in lattice constants and volume, causing structural fatigue and capacity decay. The highly oxidative Ni4+ formed at high voltages can catalyze electrolyte decomposition, resulting in the formation of a cathode electrolyte interphase (CEI) film, which consumes both electrolyte and sodium ions. Additionally, the ionic radius of Ni2+ (0.69 Å) is close to that of Na+ (1.02 Å), facilitating the detrimental migration of Ni2+ into sodium layers during cycling. This phenomenon blocks sodium-ion diffusion channels, increases impedance, and leads to capacity degradation and voltage polarization, ultimately diminishing electrochemical performance.
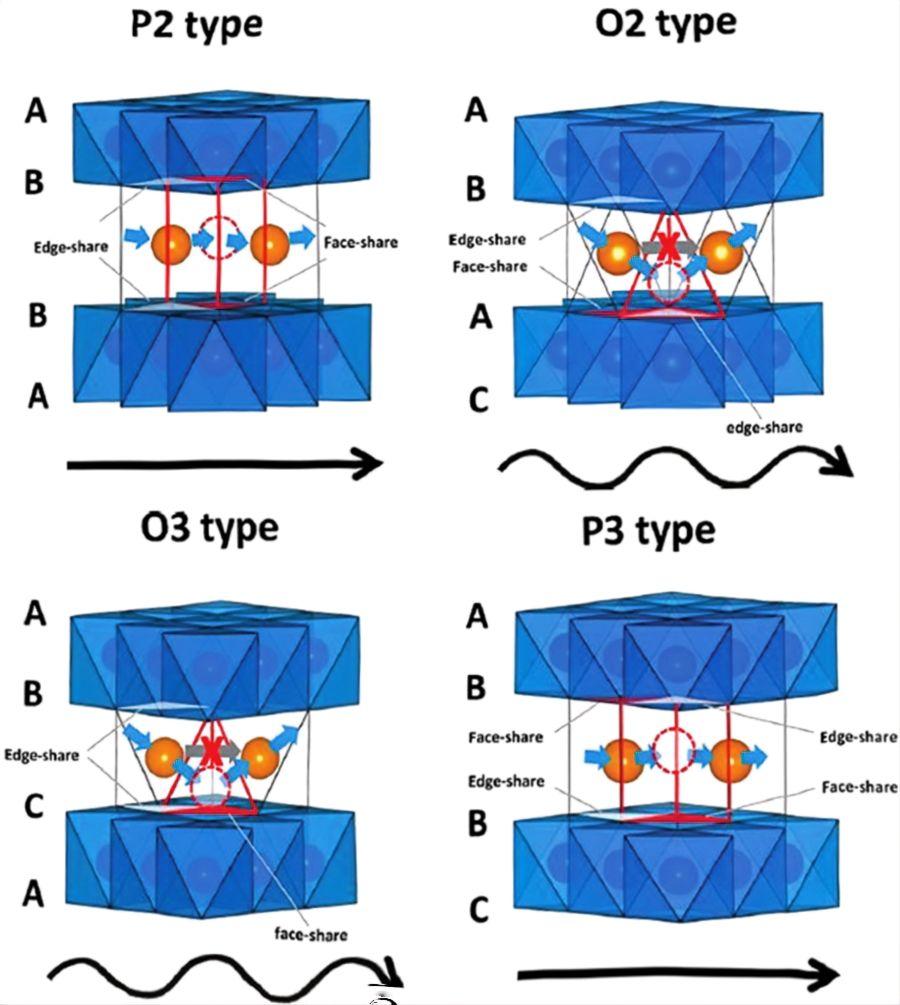
Figure 3 Schematic diagram of sodium ion migration pathways in different layered structures. A, B, and C represent different stacking sequences of oxygen atoms in the NaxMO2 framework[2]
In April 2025, CATL unveiled its Naxtra 24V integrated start-stop battery for heavy-duty trucks, designed for low-temperature operation with a service life exceeding 8 years. The total cost of ownership over its full lifecycle is 61% lower than traditional lead-acid batteries. The Naxtra truck battery also demonstrates deep discharge capability, instant starting at –40℃, and reliable activation after one year of storage, positioning it as a promising replacement for lead-acid batteries in heavy-duty applications. CATL's announced Naxtra passenger vehicle battery utilizes layered oxide cathode materials, achieving an energy density of 175 Wh/kg while maintaining 90% available capacity at –40℃. Even at an extreme state of charge (SOC) of only 10%, the battery exhibits virtually no power degradation at –40℃. Supporting peak charging rates of 5C and a range of 500 km, with over 10,000 cycles, this technology shows strong potential to lead the development of sodium-ion battery cathode materials.
Polyanionic compounds
The chemical formula of polyanionic compounds is generally expressed as NaxMy[(XOm)n–]z, where M represents transition metal elements such as Fe, Mn, or V, which enable the reversible storage and release of sodium ions, and X represents non-metal elements such as P, S, or Si. These elements, together with oxygen, form polyanionic groups and establish a stable crystal structure. A typical polyanionic compound is NaFePO4, which exhibits a structure similar to LiFePO4, as shown in Figure 4. Its theoretical specific capacity is 154 mAh/g. However, due to limitations such as slow electron and ion migration rates caused by the crystal structure, irreversible phase transitions during the initial charge-discharge cycles, interfacial side reactions, and the presence of inactive materials in the electrode, the actual specific capacity is lower than the theoretical value. With an average operating voltage of 3V, this material is suitable for applications such as low-speed electric vehicles and energy storage stations, where requirements for safety and cost-effectiveness are prioritized.
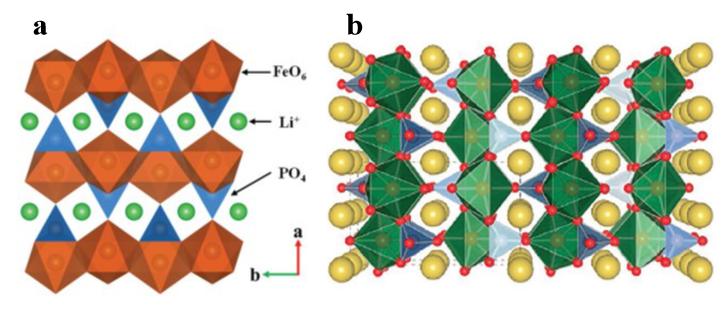
Figure 4a Structure of LiFePO4, Figure 4b Structure of NaFePO4 (FeO6 octahedra and phosphate tetrahedra are shown in green and blue respectively, with sodium atoms represented by yellow spheres)[3]
Although NaFePO4 exhibits promising performance, its synthesis methods remain challenging, necessitating further exploration and refinement of production pathways. Currently, the most commercially promising polyanionic cathode material for sodium-ion batteries is sodium iron pyrophosphate (NFPP). As a novel polyanionic cathode material with the chemical formula Na4Fe3(PO4)2P2O7, NFPP features a unique three-dimensional framework structure. Its structural diagram is shown in Figure 5. This architecture is formed by the interweaving of [P2O7]4– units with [Fe3P2O13]n layers, creating an extensive tunnel network for sodium ion transport. This characteristic makes NFPP particularly noteworthy in the field of battery materials, especially for its significant implications in enhancing battery performance and safety.
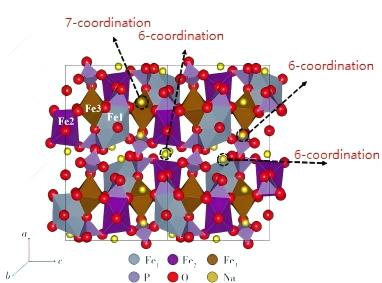
Figure 5 Crystal structure of NFPP and sodium coordination environment
The theoretical specific capacity of NFPP is 129 mAh/g, but in practice, it generally achieves only 90–100 mAh/g. This reduction is primarily due to sodium-iron site mixing, where sodium and iron ions occupy each other's positions during both synthesis and charge-discharge processes, resulting in a capacity loss of 20–30%. On September 18, 2025, EVE Lithium Energy unveiled the first large-scale 180 kWh sodium-ion battery energy storage system. The cell utilizes an improved NFPP-based material designated NF115L, which demonstrates a cycle life exceeding 30,000 cycles, a discharge power of over 1 P, and an operating temperature range from –40℃ to 60℃. It also supports ultra-long storage at 0% state of charge (SOC). With higher safety and environmental friendliness, the system achieves a 42% reduction in full lifecycle carbon emissions compared to lithium-ion batteries. Future research and development of NFPP materials will focus on addressing challenges such as low electrical conductivity, slow electron/ion migration rates, and capacity loss.
Prussian blue analogues
Prussian blue analogues (PBAs) are a class of crystalline materials with unique structures and properties. Their structure is similar to that of Prussian blue, and their general chemical formula can be expressed as AxM[M'(CN)6]y·nH2O (0<x≤2, 0<y≤1), where A represents alkali metal ions (such as Na+, K+, etc.), while M and M' denote transition metal ions. The variables x, y, and n represent the stoichiometric coefficients of different ions. Benefiting from its open three-dimensional framework, PBAs exhibit high ionic conductivity, which contributes to their excellent rate capability. Depending on the transition metal ions used (e.g., Fe, Mn), their average operating voltage can reach 3.2V–3.4V. Theoretically, when two sodium ions are extracted, a high reversible specific capacity of up to 170 mAh/g can be achieved. PBAs also feature straightforward synthesis and extremely low cost, making them seemingly ideal high-performance cathode materials for sodium-ion batteries. However, the presence of water molecules in the crystal structure not only occupies sodium sites but may also react with the electrolyte during charge and discharge processes. This side reaction consumes active sodium ions and electrolyte, generates gas, and compromises the electrode structure, ultimately leading to reduced battery cycle life. Additionally, during synthesis, [Fe(CN)6] vacancies easily form in the material, as illustrated in Figure 6.
![Schematic diagram of Prussian blue crystal structure: (a) without [Fe(CN)₆] vacancies; (b) with [Fe(CN)₆] vacancies Schematic diagram of Prussian blue crystal structure: (a) without [Fe(CN)₆] vacancies; (b) with [Fe(CN)₆] vacancies](https://www.neware.net/uploadfile/2025/0922/1758527883d05a6f.jpg)
Figure 6 Schematic diagram of Prussian blue crystal structure: (a) without [Fe(CN)₆] vacancies; (b) with [Fe(CN)₆] vacancies[4]
Vacancies not only reduce the sodium content but also tend to increase crystalline water content, further compromising the material's stability. Prussian blue analogues (PBAs) themselves exhibit a relatively porous structure with low tapping density, resulting in reduced volumetric energy density. Researchers have employed various modification methods in recent years, such as high-temperature post-synthesis treatments to reduce crystalline water content, the use of chelating agents to slow synthesis rates and minimize vacancies/crystal defects, and nanoscale engineering. These approaches have improved material performance to some extent. For example, CATL's first-generation sodium-ion battery, launched in 2021, utilized a high-capacity special Prussian blue (Prussian white) as the cathode material, achieving a cell-level energy density of 160 Wh/kg. The battery demonstrated rapid charging (reaching 80% state of charge in 15 minutes at room temperature) and maintained over 90% discharge capacity retention even at –20℃. Its system integration efficiency exceeded 80%, and its thermal stability far surpassed national safety standards. However, due to the persistent challenges associated with PBAs, coupled with the rapid development and faster commercialization of polyanionic compounds and layered oxides, research enthusiasm has gradually shifted toward LTMO (layered transition metal oxides) and NFPP (sodium iron pyrophosphate) materials. If the critical issue of crystalline water can be resolved in the future, PBA-based materials could achieve significant performance breakthroughs.
Future development of cathode materials for sodium-ion batteries
Although sodium-ion batteries hold significant cost advantages over lithium-ion batteries, their future goal is not to fully replace lithium-ion batteries but rather to complement them in specific applications where lithium-ion technology faces limitations. These include energy storage systems operating under extreme temperatures, low-speed electric vehicles, and start-stop batteries for high/low-temperature environments. Among the three major cathode material types, polyanionic compounds demonstrate exceptional stability and longevity, making them highly suitable for large-scale and grid-level energy storage systems where safety and stability are critical, but high energy density is not the primary requirement. Layered oxides, already being adopted in small electric vehicles, are poised to replace lead-acid batteries in low-speed electric vehicles, representing a substantial market opportunity. Meanwhile, Prussian blue analogues could potentially target mid-to-high-end electric vehicle markets once the key issue of crystalline water content is effectively resolved.
References
[1] Abraham K M. How comparable are sodium-ion batteries to lithium-ion counterparts?[J]. ACS Energy Letters, 2020, 5(11): 3544-3547.
[2] Liu Q N, Hu Z, Chen M Z, et al. Recent progress of layered transition metal oxide cathodes for sodium-ion batteries[J]. Small, 2019, 15(32): 1805381.
[3] Barpanda P, Lander L, Nishimura S, et al. Polyanionic insertion materials for sodium‐ion batteries[J]. Adv Energy Mater, 2018, 8(17): 1703055.
[4] Wu X, Shao M, Wu C, et al. Low defect FeFe(CN)6 framework as stable host material for high performance Li-ion batteries[J]. ACS Applied Materials & Interfaces, 2016, 8(36): 23706-23712.
Supplement: Some of the information presented above was obtained from the Internet. We are very sorry if there is any infringement! You can contact us for deletion!