With the rapid development of the new energy vehicle industry, innovation in battery technology has become a core driver of industrial upgrading. All-solid-state batteries, in particular, are widely regarded as a key direction for next-generation power batteries due to their advantages in safety, energy density, and cycle life. However, the industry has long lacked a unified technical definition of what constitutes a “true” all-solid-state battery.
On May 22, 2025, the China Society of Automotive Engineers officially released the "All-Solid-State Battery Identification Method" (T/CSAE 434—2025). While this document is established as a group standard within China, it offers valuable technical insights that may serve as a useful reference for the global battery community amid ongoing efforts to develop international standards for solid-state batteries.
1. What is an All-Solid-State Battery?
According to the technical definition outlined in the document, an all-solid-state battery refers to a battery system in which ions are transported solely by solid electrolytes between the cathode and anode. Compared with conventional liquid or gel electrolyte lithium-ion batteries, all-solid-state batteries eliminate flammable liquid electrolytes, thereby significantly enhancing safety.
2. Technical principles:breakthroughs and characterization methods
2.1 The Transition from Liquid to Solid
All-solid-state batteries (ASSBs) replace traditional liquid electrolytes with solid electrolytes, achieving breakthroughs in both energy density and safety. Their core advantages stem from innovations in electrolyte materials: sulfide electrolytes can achieve a room-temperature ionic conductivity of up to 10⁻³ S/cm, close to that of liquid electrolytes, and are compatible with lithium metal anodes (theoretical capacity: 3860 mAh/g), enabling energy densities exceeding 500 Wh/kg. Oxide electrolytes (such as LLZO) exhibit excellent thermal stability (decomposition temperature >600°C), fundamentally mitigating thermal runaway risks. However, high impedance caused by poor solid-solid interfacial contact remains a major challenge—interface contact resistance between traditional oxide electrolytes and electrodes can reach 1000 Ω·cm², requiring interface modification (e.g., LiPO₃ coating) to reduce it below 10 Ω·cm².
2.2 Key electrochemical characterization techniques
Electrochemical Impedance Spectroscopy (EIS) is a core method for analyzing interfacial properties in solid-state batteries. By applying an AC signal in the frequency range of 10 mHz to 1 MHz, a Nyquist plot can be obtained, which includes bulk resistance (Rb), interfacial charge transfer resistance (Rct), and double-layer capacitance (Cdl). Ionic conductivity is calculated using the formula:
σ = L/(Rb × A)
where L is electrolyte thickness (cm), A is electrode area (cm²), and Rb is bulk resistance (Ω). Research teams, such as one from Tsinghua University, have used this method to demonstrate that the ionic conductivity of sulfide electrolytes increases by three orders of magnitude at 50°C compared to room temperature, supporting potential high-temperature energy storage applications.
Differential Capacity Curves (dQ/dV), obtained by differentiating capacity with respect to voltage (dQ/dV = ΔQ/ΔV), reveal electrochemical reaction mechanisms. For example, characteristic peaks of NCM811 cathodes at 3.7 V and 4.0 V correspond to phase transitions during Li⁺ deintercalation. A peak shift exceeding 50 mV during cycling can serve as a quantitative indicator of capacity fade. Experimental data show that after 2,000 cycles, the decay rate of dQ/dV peak area exhibits a linear correlation with capacity retention (R² = 0.92).
High-precision battery testing systems, such as the NEWARE 4/8 Series, are designed for precise battery testing and support a wide range of applications including consumer cells, solid-state batteries, and battery materials. Featuring a four-range design, these systems offer measurement accuracy up to ±0.05% f.s., meeting precision testing requirements from microamps (μA) to milliamps (mA). With Type-C power interfaces, they provide portable battery testing solutions and support CV and EIS testing. In addition to standard charge-discharge testing, they integrate multiple functions including EIS, DCIR, CV, and pulse simulation to meet comprehensive testing needs.
3. Technical benchmark: liquid content must be less than 1%
3.1 Technical rationale for the threshold
The standard uses "weight loss rate < 1%" as a key technical indicator for identifying all-solid-state batteries, based on the difference in thermal stability between liquid and solid electrolytes. Ester-based electrolytes (e.g., EC/DMC) typically have boiling points below 150°C and can evaporate completely within 6 hours under vacuum at 120°C. In contrast, solid electrolytes (e.g., Li₇La₃Zr₂O₁₂) show a mass loss of less than 0.1% under the same conditions. Experimental data indicate that when liquid content exceeds 1%, interfacial impedance increases by more than 30% and cycle life drops below 500 cycles, validating the 1% threshold.
3.2 Key parameters of the test method
The standard test procedure includes three core steps:
(1) Preconditioning: Charge-discharge cycles are conducted at 0.1C (25°C ± 2°C, relative humidity ≤ 0.035%) until the capacity variation between two consecutive cycles is within 3%, simulating real-world usage conditions.
(2) Opening Design: Prismatic cells are opened at the explosion-proof valve (length ≥ 10% of the longest side), and pouch cells are opened at the side seal. Exposure time should not exceed 5 minutes.
(3) Vacuum Drying: Heating at 120 ± 5°C for 6 hours under a vacuum of -0.095 to -0.1 MPa. Liquid content is calculated based on weight loss:
η = (m₀ - m₁)/m₀ × 100%
where m₀ is the initial mass and m₁ is the mass after drying.
3.3 Experimental methods and equipment: from standard testing to solutions
NEWARE offers testing solutions for solid-state batteries. Its charge-discharge test equipment provides high-precision current and voltage control, enabling precise regulation of the charge-discharge process and optimization of battery performance. It can be linked with temperature chambers (temperature control systems) and battery performance testing software BTS9.0 to control the testing environment temperature and reduce the impact of temperature fluctuations on interface stability.
Solid-State Battery Mold
NEWARE solid-state battery mold is designed for solid-state battery testing. It features a simple structure, is easy to operate, and can be used inside a glove box. Made of PEEK material with high mechanical strength and corrosion resistance, it ensures durability and chemical stability. With excellent sealing, it can be used with pressure sensors. During solid-state battery manufacturing, the mold can apply specific pressure to help form contact between solid electrolytes and electrode materials, thereby improving conductivity and overall performance.
The mold plays a critical role in battery assembly by providing precise fixation, ensuring stable positioning of battery components throughout charge-discharge cycles, significantly reducing the risk of displacement and damage, and enhancing overall battery performance and reliability. When used with an all-in-one tester, it creates a constant-temperature environment to ensure testing consistency or enables performance tests at different temperatures, improving testing efficiency and repeatability.
All-in-One Battery Testing System
NEWARE all-in-one battery testing system integrate charge-discharge testing with temperature control, allowing simultaneous constant-temperature or high-low temperature tests during battery testing. This comprehensive approach evaluates the performance and thermal stability of solid-state batteries under various temperature conditions, providing critical data for high-temperature tolerance or low-temperature startup capability, thereby supporting the advancement of solid-state battery technology in extreme environments.
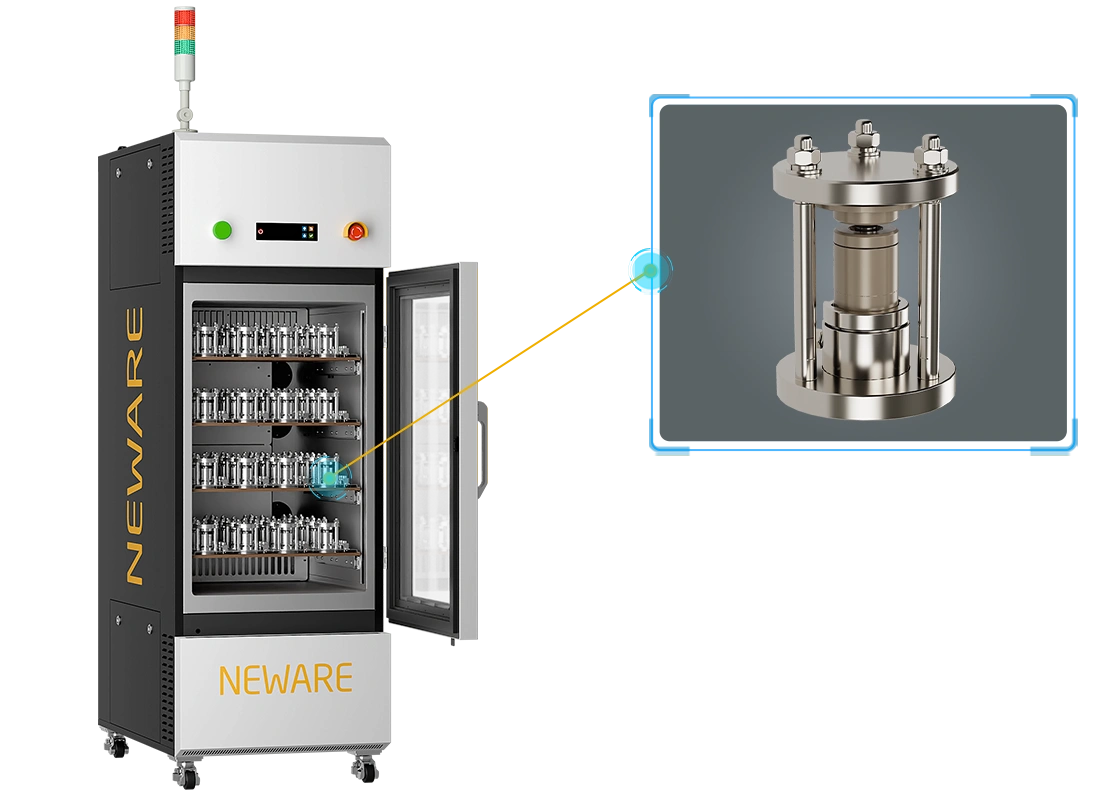
NEWARE Solid-State Battery Mold & All-in-One Battery Testing System
Integrated Design: Combining charge-discharge testing and temperature control functions simplifies laboratory testing processes, reduces wiring complexity, and provides a more efficient testing environment.
Temperature Control: Precise temperature control ensures consistent and repeatable experimental conditions. Battery cycle life can be evaluated under set temperature cycling conditions.
Broader Industry Implications
Previously, various products marketed as "solid-state batteries" have emerged, employing diverse technical routes (e.g., semi-solid, quasi-solid, all-solid), making it difficult for consumers and automakers to discern their true performance.
The introduction of a clear technical standard helps regulate market claims, prevent misleading promotion of “pseudo-solid-state” batteries, guide R&D efforts toward genuine all-solid-state technology, and foster industry chain collaboration by providing a unified evaluation framework for material, cell, and vehicle manufacturers.
Challenges and future of All-Solid-State Batteries
Despite their promising prospects, the industrialization of all-solid-state batteries still faces challenges such as high interfacial impedance, high manufacturing costs, and insufficient fast-charging performance. The release of this standard not only provides technical clarity but also offers valuable guidance for global R&D directions.
Looking ahead, with continued advancements in material innovation (e.g., sulfide, oxide electrolytes) and manufacturing processes, all-solid-state batteries are expected to see commercial application in high-end electric vehicles and energy storage systems.